Ablation of the transcriptional regulator Id1 enhances energy expenditure, increases insulin sensitivity, and protects against age and diet induced insulin resistance, and hepatosteatosis
- PMID: 21990377
- PMCID: PMC3250238
- DOI: 10.1096/fj.11-190892
Ablation of the transcriptional regulator Id1 enhances energy expenditure, increases insulin sensitivity, and protects against age and diet induced insulin resistance, and hepatosteatosis
Abstract
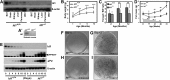
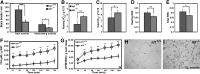
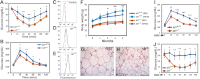
Obesity is a major health concern that contributes to the development of diabetes, hyperlipidemia, coronary artery disease, and cancer. Id proteins are helix-loop-helix transcription factors that regulate the proliferation and differentiation of cells from multiple tissues, including adipocytes. We screened mouse tissues for the expression of Id1 and found that Id1 protein is highly expressed in brown adipose tissue (BAT) and white adipose tissue (WAT), suggesting a role for Id1 in adipogenesis and cell metabolism. Id1(-/-) mice are viable but show a significant reduction in fat mass (P<0.005) over the life of the animal that was not due to decreased number of adipocytes. Analysis of Id1(-/-) mice revealed higher energy expenditure, increased lipolysis, and fatty acid oxidation, resulting in reduced triglyceride accumulation in WAT compared to Id1(+/+) mice. Serum levels of triglycerides (193.9±32.2 vs. 86.5±33.8, P<0.0005), cholesterol (189.4±33.8 vs. 110.6±8.23, P<0.0005) and leptin (1263±835 vs. 222±260, P<0.005) were significantly lower in aged Id1(-/-) mice compared to Id1(+/+) mice. Id1-deficient mice have higher resting (P<0.005) and total (P<0.05) O(2) consumption and lower respiratory exchange ratio (P<0.005), confirming that Id1(-/-) mice use a higher proportion of lipid as an energy source for the increased energy expenditure. The expression of PGC1α and UCP1 were 2- to 3-fold up-regulated in Id1(-/-) BAT, suggesting that loss of Id1 increases thermogenesis. As a consequence of higher energy expenditure and reduced fat mass, Id1(-/-) mice displayed enhanced insulin sensitivity. Id1 deficiency protected mice against age- and high-fat-diet-induced adiposity, insulin resistance, and hepatosteatosis. Our findings suggest that Id1 plays a critical role in the regulation of energy homeostasis and could be a potential target in the treatment of insulin resistance and fatty liver disease.
Figures


References
-
- Spiegelman B. M., Flier J. S. (1996) Adipogenesis and obesity: rounding out the big picture. Cell 87, 377–389 - PubMed
-
- Klaus S. (1997) Functional differentiation of white and brown adipocytes. Bioessays 19, 215–223 - PubMed
-
- Galic S., Oakhill J. S., Steinberg G. R. (2010) Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 316, 129–139 - PubMed
-
- Ricquier D. (2005) Respiration uncoupling and metabolism in the control of energy expenditure. Proc. Nutr. Soc. 64, 47–52 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

