RSPO1/β-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary
- PMID: 21991325
- PMCID: PMC3185015
- DOI: 10.1371/journal.pone.0025641
RSPO1/β-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary
Abstract
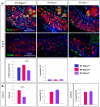
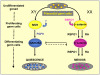
Differentiation of germ cells into male gonocytes or female oocytes is a central event in sexual reproduction. Proliferation and differentiation of fetal germ cells depend on the sex of the embryo. In male mouse embryos, germ cell proliferation is regulated by the RNA helicase Mouse Vasa homolog gene and factors synthesized by the somatic Sertoli cells promote gonocyte differentiation. In the female, ovarian differentiation requires activation of the WNT/β-catenin signaling pathway in the somatic cells by the secreted protein RSPO1. Using mouse models, we now show that Rspo1 also activates the WNT/β-catenin signaling pathway in germ cells. In XX Rspo1(-/-) gonads, germ cell proliferation, expression of the early meiotic marker Stra8, and entry into meiosis are all impaired. In these gonads, impaired entry into meiosis and germ cell sex reversal occur prior to detectable Sertoli cell differentiation, suggesting that β-catenin signaling acts within the germ cells to promote oogonial differentiation and entry into meiosis. Our results demonstrate that RSPO1/β-catenin signaling is involved in meiosis in fetal germ cells and contributes to the cellular decision of germ cells to differentiate into oocyte or sperm.
Conflict of interest statement
Figures







References
-
- Ewen KA, Koopman P. Mouse germ cell development: from specification to sex determination. Mol Cell Endocrinol. 2010;323:76–93. - PubMed
-
- Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009;25:19–29. - PubMed
-
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. - PubMed
-
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, et al. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

