High macroalgal cover and low coral recruitment undermines the potential resilience of the world's southernmost coral reef assemblages
- PMID: 21991366
- PMCID: PMC3185058
- DOI: 10.1371/journal.pone.0025824
High macroalgal cover and low coral recruitment undermines the potential resilience of the world's southernmost coral reef assemblages
Abstract
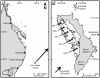
Coral reefs are under increasing pressure from anthropogenic and climate-induced stressors. The ability of reefs to reassemble and regenerate after disturbances (i.e., resilience) is largely dependent on the capacity of herbivores to prevent macroalgal expansion, and the replenishment of coral populations through larval recruitment. Currently there is a paucity of this information for higher latitude, subtropical reefs. To assess the potential resilience of the benthic reef assemblages of Lord Howe Island (31°32'S, 159°04'E), the worlds' southernmost coral reef, we quantified the benthic composition, densities of juvenile corals (as a proxy for coral recruitment), and herbivorous fish communities. Despite some variation among habitats and sites, benthic communities were dominated by live scleractinian corals (mean cover 37.4%) and fleshy macroalgae (20.9%). Live coral cover was higher than in most other subtropical reefs and directly comparable to lower latitude tropical reefs. Juvenile coral densities (0.8 ind.m(-2)), however, were 5-200 times lower than those reported for tropical reefs. Overall, macroalgal cover was negatively related to the cover of live coral and the density of juvenile corals, but displayed no relationship with herbivorous fish biomass. The biomass of herbivorous fishes was relatively low (204 kg.ha(-1)), and in marked contrast to tropical reefs was dominated by macroalgal browsing species (84.1%) with relatively few grazing species. Despite their extremely low biomass, grazing fishes were positively related to both the density of juvenile corals and the cover of bare substrata, suggesting that they may enhance the recruitment of corals through the provision of suitable settlement sites. Although Lord Howe Islands' reefs are currently coral-dominated, the high macroalgal cover, coupled with limited coral recruitment and low coral growth rates suggest these reefs may be extremely susceptible to future disturbances.
Conflict of interest statement
Figures




References
-
- Bellwood DR, Hughes TP, Folke C, Nyström M. Confronting the coral reef crisis. Nature. 2004;429:827–833. - PubMed
-
- Nyström M, Folke C. Spatial resilience of coral reefs. Ecosystems. 2001;4:406–417.
-
- Sheppard CRC. Predicted recurrences of coral mortality in the Indian Ocean. Nature. 2003;425:294–297. - PubMed
-
- Donner SD, Skirving WJ, Little CM, Hoegh-Guldberg O, Oppenheimer M. Global assessment of coral bleaching and required rates of adaptation under climate change. Glob Change Biol. 2005;11:2251–2265. - PubMed
-
- Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

