Betanodavirus induces oxidative stress-mediated cell death that prevented by anti-oxidants and zfcatalase in fish cells
- PMID: 21991373
- PMCID: PMC3185053
- DOI: 10.1371/journal.pone.0025853
Betanodavirus induces oxidative stress-mediated cell death that prevented by anti-oxidants and zfcatalase in fish cells
Abstract
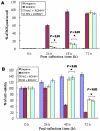
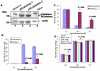
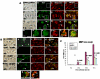
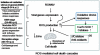
The role of oxidative stress in the pathogenesis of RNA nervous necrosis virus infection is still unknown. Red-spotted grouper nervous necrosis virus (RGNNV) induced free radical species (ROS) production at 12-24 h post-infection (pi; early replication stage) in fish GF-1 cells, and then at middle replication stage (24-48 h pi), this ROS signal may upregulate some expressions of the anti-oxidant enzymes Cu/Zn SOD and catalase, and eventually expression of the transcription factor Nrf2. Furthermore, both antioxidants diphenyliodonium and N-acetylcysteine or overexpression of zebrafish catalase in GF-1 cells also reduced ROS production and protected cells for enhancing host survival rate due to RGNNV infection. Furthermore, localization of ROS production using esterase activity and Mitotracker staining assays found that the ROS generated can affect mitochondrial morphology changes and causes ΔΨ loss, both of which can be reversed by antioxidant treatment. Taken together, our data suggest that RGNNV induced oxidative stress response for playing dual role that can initiate the host oxidative stress defense system to upregulate expression of antioxidant enzymes and induces cell death via disrupting the mitochondrial morphology and inducing ΔΨ loss, which can be reversed by anti-oxidants and zfcatalase, which provide new insight into betanodavirus-induced ROS-mediated pathogenesis.
Conflict of interest statement
Figures



References
-
- Ball L, Johnson K. Reverse genetics of nodaviruses. Adv Virus Res. 1999;53:229–244. - PubMed
-
- Bovo G, Nishizawa T, Maltese C, Borghesan F, Mutinelli F, et al. Viral encephalopathy and retinopathy of farmed marine fish species in Italy. Virus Res. 1999;63:143–146. - PubMed
-
- Munday B, Kwang J, Moody N. Betanodavirus infections of teleost fish: a review. J Fish Dis. 2002;25:127–142.
-
- Schneemann A, Reddy V, Johnson J. The structure and function of nodavirus particles: a paradigm for understanding chemical biology. Adv Virus Res. 1998;50:381–446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

