Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway
- PMID: 21991382
- PMCID: PMC3186800
- DOI: 10.1371/journal.pone.0025900
Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway
Abstract
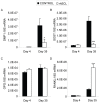
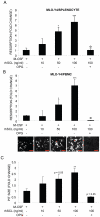
Sclerostin is a product of mature osteocytes embedded in mineralised bone and is a negative regulator of bone mass and osteoblast differentiation. While evidence suggests that sclerostin has an anti-anabolic role, the possibility also exists that sclerostin has catabolic activity. To test this we treated human primary pre-osteocyte cultures, cells we have found are exquisitely sensitive to sclerostin, or mouse osteocyte-like MLO-Y4 cells, with recombinant human sclerostin (rhSCL) and measured effects on pro-catabolic gene expression. Sclerostin dose-dependently up-regulated the expression of receptor activator of nuclear factor kappa B (RANKL) mRNA and down-regulated that of osteoprotegerin (OPG) mRNA, causing an increase in the RANK:OPG mRNA ratio. To examine the effects of rhSCL on resulting osteoclastic activity, MLO-Y4 cells plated onto a bone-like substrate were primed with rhSCL for 3 days and then either mouse splenocytes or human peripheral blood mononuclear cells (PBMC) were added. This resulted in cultures with elevated osteoclastic resorption (approximately 7-fold) compared to untreated co-cultures. The increased resorption was abolished by co-addition of recombinant OPG. In co-cultures of MLO-Y4 cells with PBMC, SCL also increased the number and size of the TRAP-positive multinucleated cells formed. Importantly, rhSCL had no effect on TRAP-positive cell formation from monocultures of either splenocytes or PBMC. Further, rhSCL did not induce apoptosis of MLO-Y4 cells, as determined by caspase activity assays, demonstrating that the osteoclastic response was not driven by dying osteocytes. Together, these results suggest that sclerostin may have a catabolic action through promotion of osteoclast formation and activity by osteocytes, in a RANKL-dependent manner.
Conflict of interest statement
Figures



References
-
- Baron R, Rawadi G, Roman-Roman S. Wnt signaling: a key regulator of bone mass. Curr Top Dev Biol. 2006;76:103–127. - PubMed
-
- Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, et al. Targeted Deletion of the Sclerostin Gene in Mice Results in Increased Bone Formation and Bone Strength. J Bone Miner Res. 2008;23:860–869. - PubMed
-
- ten Dijke P, Krause C, de Gorter DJ, Lowik CW, van Bezooijen RL. Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signaling. J Bone Joint Surg Am. 2008;90(Suppl 1):31–35. - PubMed
-
- Staehling-Hampton K, Proll S, Paeper BW, Zhao L, Charmley P, et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet. 2002;110:144–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

