Effects of incomplete decay in fluorescence lifetime estimation
- PMID: 21991544
- PMCID: PMC3184861
- DOI: 10.1364/BOE.2.002517
Effects of incomplete decay in fluorescence lifetime estimation
Abstract
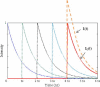
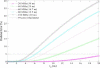
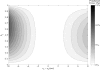
Fluorescence lifetime imaging has emerged as an important microscopy technique, where high repetition rate lasers are the primary light sources. As fluorescence lifetime becomes comparable to intervals between consecutive excitation pulses, incomplete fluorescence decay from previous pulses can superimpose onto the subsequent decay measurements. Using a mathematical model, the incomplete decay effect has been shown to lead to overestimation of the amplitude average lifetime except in mono-exponential decays. An inverse model is then developed to correct the error from this effect and the theoretical simulations are tested by experimental results.
Keywords: (170.3650) Lifetime-based sensing; 180.2520 Fluorescence microscopy.
Figures

References
-
- Kelleher M. T., Fruhwirth G., Patel G., Ofo E., Festy F., Barber P. R., Ameer-Beg S. M., Vojnovic B., Gillett C., Coolen A., Kéri G., Ellis P. A., Ng T., “The potential of optical proteomic technologies to individualize prognosis and guide rational treatment for cancer patients,” Target Oncol 4(3), 235–252 (2009). 10.1007/s11523-009-0116-y - DOI - PMC - PubMed
-
- Munro I., McGinty J., Galletly N., Requejo-Isidro J., Lanigan P. M. P., Elson D. S., Dunsby C., Neil M. A. A., Lever M. J., Stamp G. W. H., French P. M. W., “Toward the clinical application of time-domain fluorescence lifetime imaging,” J. Biomed. Opt. 10(5), 051403 (2005). 10.1117/1.2102807 - DOI - PubMed
-
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, 3rd ed. (Kluwer Academic/Plenum, New York, 2006), Chap. 4.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
