Adjuvant trastuzumab in HER2-positive breast cancer
- PMID: 21991949
- PMCID: PMC3268553
- DOI: 10.1056/NEJMoa0910383
Adjuvant trastuzumab in HER2-positive breast cancer
Abstract
Background: Trastuzumab improves survival in the adjuvant treatment of HER-positive breast cancer, although combined therapy with anthracycline-based regimens has been associated with cardiac toxicity. We wanted to evaluate the efficacy and safety of a new nonanthracycline regimen with trastuzumab.
Methods: We randomly assigned 3222 women with HER2-positive early-stage breast cancer to receive doxorubicin and cyclophosphamide followed by docetaxel every 3 weeks (AC-T), the same regimen plus 52 weeks of trastuzumab (AC-T plus trastuzumab), or docetaxel and carboplatin plus 52 weeks of trastuzumab (TCH). The primary study end point was disease-free survival. Secondary end points were overall survival and safety.
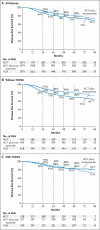
Results: At a median follow-up of 65 months, 656 events triggered this protocol-specified analysis. The estimated disease-free survival rates at 5 years were 75% among patients receiving AC-T, 84% among those receiving AC-T plus trastuzumab, and 81% among those receiving TCH. Estimated rates of overall survival were 87%, 92%, and 91%, respectively. No significant differences in efficacy (disease-free or overall survival) were found between the two trastuzumab regimens, whereas both were superior to AC-T. The rates of congestive heart failure and cardiac dysfunction were significantly higher in the group receiving AC-T plus trastuzumab than in the TCH group (P<0.001). Eight cases of acute leukemia were reported: seven in the groups receiving the anthracycline-based regimens and one in the TCH group subsequent to receiving an anthracycline outside the study.
Conclusions: The addition of 1 year of adjuvant trastuzumab significantly improved disease-free and overall survival among women with HER2-positive breast cancer. The risk-benefit ratio favored the nonanthracycline TCH regimen over AC-T plus trastuzumab, given its similar efficacy, fewer acute toxic effects, and lower risks of cardiotoxicity and leukemia. (Funded by Sanofi-Aventis and Genentech; BCIRG-006 ClinicalTrials.gov number, NCT00021255.).
Figures

Comment in
-
Steady progress against HER2-positive breast cancer.N Engl J Med. 2011 Oct 6;365(14):1336-8. doi: 10.1056/NEJMe1101326. N Engl J Med. 2011. PMID: 21991956 No abstract available.
-
Adjuvant trastuzumab in HER2-positive breast cancer.N Engl J Med. 2012 Feb 16;366(7):663; author reply 664-6. doi: 10.1056/NEJMc1112823. N Engl J Med. 2012. PMID: 22335747 No abstract available.
-
Adjuvant trastuzumab in HER2-positive breast cancer.N Engl J Med. 2012 Feb 16;366(7):663-4; author reply 664-6. doi: 10.1056/NEJMc1112823. N Engl J Med. 2012. PMID: 22335748 No abstract available.
-
Adjuvant trastuzumab in HER2-positive breast cancer.N Engl J Med. 2012 Feb 16;366(7):664; author reply 664-6. doi: 10.1056/NEJMc1112823. N Engl J Med. 2012. PMID: 22335749 No abstract available.
-
Adjuvant trastuzumab in HER2-positive breast cancer.N Engl J Med. 2012 Feb 16;366(7):664; author reply 664-6. doi: 10.1056/NEJMc1112823. N Engl J Med. 2012. PMID: 22335750 No abstract available.
References
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-onco-gene in human breast and ovarian cancer. Science. 1989;244:707–12. - PubMed
-
- Arboleda MJ, Lyons JF, Kabbinavar FF, et al. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196–206. - PubMed
-
- Benz CC, Scott GK, Sarup JC, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24:85–95. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
