Activating and deactivating roles of lipid bilayers on the Ca(2+)-ATPase/phospholamban complex
- PMID: 21992175
- PMCID: PMC3487395
- DOI: 10.1021/bi200759y
Activating and deactivating roles of lipid bilayers on the Ca(2+)-ATPase/phospholamban complex
Abstract
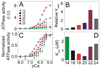
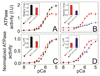
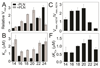
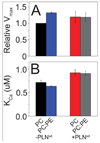
The physicochemical properties of the lipid bilayer shape the structure and topology of membrane proteins and regulate their biological function. Here, we investigated the functional effects of various lipid bilayer compositions on the sarcoplasmic reticulum (SR) Ca(2+)-ATPase (SERCA) in the presence and absence of its endogenous regulator, phospholamban (PLN). In the cardiac muscle, SERCA hydrolyzes one ATP molecule to translocate two Ca(2+) ions into the SR membrane per enzymatic cycle. Unphosphorylated PLN reduces SERCA's affinity for Ca(2+) and affects the enzymatic turnover. We varied bilayer thickness, headgroup, and fluidity and found that both the maximal velocity (V(max)) of the enzyme and its apparent affinity for Ca(2+) (K(Ca)) are strongly affected. Our results show that (a) SERCA's V(max) has a biphasic dependence on bilayer thickness, reaching maximum activity with 22-carbon lipid chain length, (b) phosphatidylethanolamine (PE) and phosphatidylserine (PS) increase Ca(2+) affinity, and (c) monounsaturated lipids afford higher SERCA V(max) and Ca(2+) affinity than diunsaturated lipids. The presence of PLN removes the activating effect of PE and shifts SERCA's activity profile, with a maximal activity reached in bilayers with 20-carbon lipid chain length. Our results in synthetic lipid systems compare well with those carried out in native SR lipids. Importantly, we found that specific membrane compositions closely reproduce PLN effects (V(max) and K(Ca)) found in living cells, reconciling an ongoing controversy regarding the regulatory role of PLN on SERCA function. Taken with the physiological changes occurring in the SR membrane composition, these studies underscore a possible allosteric role of the lipid bilayers on the SERCA/PLN complex.
Figures


Similar articles
-
Allosteric regulation of SERCA by phosphorylation-mediated conformational shift of phospholamban.Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17338-43. doi: 10.1073/pnas.1303006110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101520 Free PMC article.
-
Conformational memory in the association of the transmembrane protein phospholamban with the sarcoplasmic reticulum calcium pump SERCA.J Biol Chem. 2017 Dec 29;292(52):21330-21339. doi: 10.1074/jbc.M117.794453. Epub 2017 Oct 29. J Biol Chem. 2017. PMID: 29081402 Free PMC article.
-
Pathological mutations in the phospholamban cytoplasmic region affect its topology and dynamics modulating the extent of SERCA inhibition.Biochim Biophys Acta Biomembr. 2024 Oct;1866(7):184370. doi: 10.1016/j.bbamem.2024.184370. Epub 2024 Jul 8. Biochim Biophys Acta Biomembr. 2024. PMID: 38986894
-
The SarcoEndoplasmic Reticulum Calcium ATPase.Subcell Biochem. 2018;87:229-258. doi: 10.1007/978-981-10-7757-9_8. Subcell Biochem. 2018. PMID: 29464562 Review.
-
Phospholamban and sarcolipin: Are they functionally redundant or distinct regulators of the Sarco(Endo)Plasmic Reticulum Calcium ATPase?J Mol Cell Cardiol. 2016 Feb;91:81-91. doi: 10.1016/j.yjmcc.2015.12.030. Epub 2015 Dec 29. J Mol Cell Cardiol. 2016. PMID: 26743715 Free PMC article. Review.
Cited by
-
Skeletal muscle phosphatidylcholine and phosphatidylethanolamine are related to insulin sensitivity and respond to acute exercise in humans.J Appl Physiol (1985). 2016 Jun 1;120(11):1355-63. doi: 10.1152/japplphysiol.00664.2015. Epub 2016 Mar 31. J Appl Physiol (1985). 2016. PMID: 27032901 Free PMC article.
-
Hydrophobic imbalance in the cytoplasmic domain of phospholamban is a determinant for lethal dilated cardiomyopathy.J Biol Chem. 2012 May 11;287(20):16521-9. doi: 10.1074/jbc.M112.360859. Epub 2012 Mar 16. J Biol Chem. 2012. PMID: 22427649 Free PMC article.
-
Skeletal Muscle Phospholipid Metabolism Regulates Insulin Sensitivity and Contractile Function.Diabetes. 2016 Feb;65(2):358-70. doi: 10.2337/db15-0659. Epub 2015 Oct 28. Diabetes. 2016. PMID: 26512026 Free PMC article.
-
Housing Temperature Impacts the Systemic and Tissue-Specific Molecular Responses to Cancer in Mice.J Cachexia Sarcopenia Muscle. 2025 Apr;16(2):e13781. doi: 10.1002/jcsm.13781. J Cachexia Sarcopenia Muscle. 2025. PMID: 40237521 Free PMC article.
-
Glycerophospholipids in ALS: insights into disease mechanisms and clinical implication.Mol Neurodegener. 2025 Jul 26;20(1):85. doi: 10.1186/s13024-025-00876-3. Mol Neurodegener. 2025. PMID: 40713843 Free PMC article. Review.
References
-
- Bers DM. Cardiac Excitation-Contraction Coupling. Nature. 2002;415:198–205. - PubMed
-
- Brini M, Carafoli E. Calcium Pumps in Health and Disease. Physiol. Rev. 2009;89:1341–1378. - PubMed
-
- Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR. Sequence Analysis of Phospholamban. Identification of Phosphorylation Sites and Two Major Structural Domains. J. Biol. Chem. 1986;261:13333–13341. - PubMed
-
- Wegener AD, Simmerman HK, Lindemann JP, Jones LR. Phospholamban Phosphorylation in Intact Ventricles. Phosphorylation of Serine 16 and Threonine 17 in Response to Beta-Adrenergic Stimulation. J. Biol. Chem. 1989;264:11468–11474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

