Activating and deactivating roles of lipid bilayers on the Ca(2+)-ATPase/phospholamban complex
- PMID: 21992175
- PMCID: PMC3487395
- DOI: 10.1021/bi200759y
Activating and deactivating roles of lipid bilayers on the Ca(2+)-ATPase/phospholamban complex
Abstract
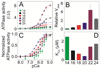
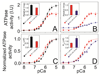
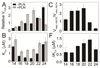
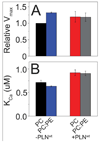
The physicochemical properties of the lipid bilayer shape the structure and topology of membrane proteins and regulate their biological function. Here, we investigated the functional effects of various lipid bilayer compositions on the sarcoplasmic reticulum (SR) Ca(2+)-ATPase (SERCA) in the presence and absence of its endogenous regulator, phospholamban (PLN). In the cardiac muscle, SERCA hydrolyzes one ATP molecule to translocate two Ca(2+) ions into the SR membrane per enzymatic cycle. Unphosphorylated PLN reduces SERCA's affinity for Ca(2+) and affects the enzymatic turnover. We varied bilayer thickness, headgroup, and fluidity and found that both the maximal velocity (V(max)) of the enzyme and its apparent affinity for Ca(2+) (K(Ca)) are strongly affected. Our results show that (a) SERCA's V(max) has a biphasic dependence on bilayer thickness, reaching maximum activity with 22-carbon lipid chain length, (b) phosphatidylethanolamine (PE) and phosphatidylserine (PS) increase Ca(2+) affinity, and (c) monounsaturated lipids afford higher SERCA V(max) and Ca(2+) affinity than diunsaturated lipids. The presence of PLN removes the activating effect of PE and shifts SERCA's activity profile, with a maximal activity reached in bilayers with 20-carbon lipid chain length. Our results in synthetic lipid systems compare well with those carried out in native SR lipids. Importantly, we found that specific membrane compositions closely reproduce PLN effects (V(max) and K(Ca)) found in living cells, reconciling an ongoing controversy regarding the regulatory role of PLN on SERCA function. Taken with the physiological changes occurring in the SR membrane composition, these studies underscore a possible allosteric role of the lipid bilayers on the SERCA/PLN complex.
Figures


References
-
- Bers DM. Cardiac Excitation-Contraction Coupling. Nature. 2002;415:198–205. - PubMed
-
- Brini M, Carafoli E. Calcium Pumps in Health and Disease. Physiol. Rev. 2009;89:1341–1378. - PubMed
-
- Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR. Sequence Analysis of Phospholamban. Identification of Phosphorylation Sites and Two Major Structural Domains. J. Biol. Chem. 1986;261:13333–13341. - PubMed
-
- Wegener AD, Simmerman HK, Lindemann JP, Jones LR. Phospholamban Phosphorylation in Intact Ventricles. Phosphorylation of Serine 16 and Threonine 17 in Response to Beta-Adrenergic Stimulation. J. Biol. Chem. 1989;264:11468–11474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

