Heat shock protein 70/peptide complexes: potent mediators for the generation of antiviral T cells particularly with regard to low precursor frequencies
- PMID: 21992180
- PMCID: PMC3217864
- DOI: 10.1186/1479-5876-9-175
Heat shock protein 70/peptide complexes: potent mediators for the generation of antiviral T cells particularly with regard to low precursor frequencies
Abstract
Background: Heat shock protein 70 (HSP70) has gained major attention as an adjuvant capable of inducing antigen-specific CD8(+) and CD4(+) T-cell responses. The ability of HSP70/peptide complexes to elicit cytotoxic T-cell (CTL) responses by cross-presentation of exogenous antigens via HLA class I molecules is of central interest in immunotherapy. We examined the role of HSP70/CMVpp65(495-503)-peptide complex (HSP70/CMV-PC) in HLA class I-restricted cross-presentation for ex vivo expansion of CMV-specific CTLs.
Methods: CMV-specific T cells generated from PBMCs of HLA-A*02:01/CMV-seropositive donors were stimulated for 21 days with HSP70/CMV-PC and analyzed in functional assays. As a control PBMCs were cultured in the presence of CMVpp65(495-503) peptide or HSP70. Increase of CMV-specific CTLs was visualized by pentameric HLA-A*02:01/CMVpp65(495-503) complex.
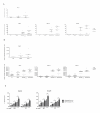
Results: About 90% of HSP70/CMV-PC generated T cells were CMV-specific and exhibited significantly higher IFN-γ secretion, cytotoxic activity, and an increased heme oxygenase 1 (HO-1) gene expression as compared to about 69% of those stimulated with CMVpp65(495-503) peptide. We decided to classify the HLA-A*02:01/CMV-seropositive donors as weak, medium, and strong responder according to the frequency of generated A2/CMV-pentamer-positive CD8(+) T cells. HSP70/CMV-PC significantly induces strong antiviral T-cell responses especially in those donors with low memory precursor frequencies. Blockage of CD91 with α2-macroglobulin markedly reduced proliferation of antiviral T cells suggesting a major role of this receptor in the uptake of HSP70/CMV-PC.
Conclusion: This study clearly demonstrates that HSP70/CMV-PC is a potent mediator to induce stronger T-cell responses compared to antiviral peptides. This simple and efficient technique may help to generate significant quantities of antiviral CTLs by cross-presentation. Thus, we propose HSP70 for chaperoning peptides to reach an efficient level of cross-presentation. HSP70/peptide complexes may be particularly useful to generate stronger T-cell responses in cases of low precursor frequencies and may help to improve the efficiency of antigen-specific T-cell therapy for minor antigens.
Figures
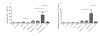

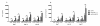
Similar articles
-
The matrix protein pp65(341-350): a peptide that induces ex vivo stimulation and in vitro expansion of CMV-specific CD8+ T cells in subjects bearing either HLA-A*2402 or A*0101 allele.Transfusion. 2003 Nov;43(11):1567-74. doi: 10.1046/j.1537-2995.2003.00564.x. Transfusion. 2003. PMID: 14617317
-
Soluble recombinant CMVpp65 spanning multiple HLA alleles for reconstitution of antiviral CD4+ and CD8+ T-cell responses after allogeneic stem cell transplantation.J Immunother. 2010 Jan;33(1):60-72. doi: 10.1097/CJI.0b013e3181b56dcc. J Immunother. 2010. PMID: 19952955
-
Evaluation of suitable target antigens and immunoassays for high-accuracy immune monitoring of cytomegalovirus and Epstein-Barr virus-specific T cells as targets of interest in immunotherapeutic approaches.J Immunol Methods. 2014 Jun;408:101-13. doi: 10.1016/j.jim.2014.05.011. Epub 2014 May 28. J Immunol Methods. 2014. PMID: 24877879
-
Characterization of cytomegalovirus pp65-HLA-A24 peptide-specific CTL lines from metastatic melanoma patients.Oncol Rep. 2009 Jul;22(1):185-91. doi: 10.3892/or_00000423. Oncol Rep. 2009. PMID: 19513522
-
Heat shock protein 70: role in antigen presentation and immune stimulation.Int J Hyperthermia. 2002 Nov-Dec;18(6):563-75. doi: 10.1080/02656730210166140. Int J Hyperthermia. 2002. PMID: 12537755 Review.
Cited by
-
Expression of heat shock protein 70 in nasopharyngeal carcinomas: different expression patterns correlate with distinct clinical prognosis.J Transl Med. 2012 May 16;10:96. doi: 10.1186/1479-5876-10-96. J Transl Med. 2012. PMID: 22591702 Free PMC article.
-
HSP70L1-mediated intracellular priming of dendritic cell vaccination induces more potent CTL response against cancer.Cell Mol Immunol. 2018 Feb;15(2):135-145. doi: 10.1038/cmi.2016.33. Epub 2016 Jun 27. Cell Mol Immunol. 2018. PMID: 27345726 Free PMC article.
-
CD91-Dependent Modulation of Immune Responses by Heat Shock Proteins: A Role in Autoimmunity.Autoimmune Dis. 2012;2012:863041. doi: 10.1155/2012/863041. Epub 2012 Nov 19. Autoimmune Dis. 2012. PMID: 23209886 Free PMC article.
-
hsp70-dependent antiviral immunity against cytopathic neuronal infection by vesicular stomatitis virus.J Virol. 2013 Oct;87(19):10668-78. doi: 10.1128/JVI.00872-13. Epub 2013 Jul 24. J Virol. 2013. PMID: 23885078 Free PMC article.
-
Establishment of tumor-associated immunity requires interaction of heat shock proteins with CD91.Cancer Immunol Res. 2014 Mar;2(3):217-28. doi: 10.1158/2326-6066.CIR-13-0132. Epub 2013 Dec 31. Cancer Immunol Res. 2014. PMID: 24778318 Free PMC article.
References
-
- Calderwood SK, Gong J, Theriault JR, Mambula SS, Gray PJ Jr. Cell stress proteins: novel immunotherapeutics. Novartis Found Symp. 2008;291:115–131. discussion 131-140. - PubMed
-
- Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 2009;182:1449–1459. - PubMed
-
- Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, Parmiani G, Tosti G, Kirkwood JM, Hoos A. et al.Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol. 2008;26:955–962. doi: 10.1200/JCO.2007.11.9941. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

