Mercedes-Benz water molecules near hydrophobic wall: integral equation theories vs Monte Carlo simulations
- PMID: 21992334
- PMCID: PMC3206897
- DOI: 10.1063/1.3644934
Mercedes-Benz water molecules near hydrophobic wall: integral equation theories vs Monte Carlo simulations
Abstract
Associative version of Henderson-Abraham-Barker theory is applied for the study of Mercedes-Benz model of water near hydrophobic surface. We calculated density profiles and adsorption coefficients using Percus-Yevick and soft mean spherical associative approximations. The results are compared with Monte Carlo simulation data. It is shown that at higher temperatures both approximations satisfactory reproduce the simulation data. For lower temperatures, soft mean spherical approximation gives good agreement at low and at high densities while in at mid range densities, the prediction is only qualitative. The formation of a depletion layer between water and hydrophobic surface was also demonstrated and studied.
© 2011 American Institute of Physics
Figures


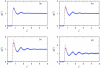
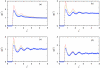




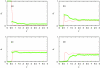

References
-
- Torrie G. M., Kusalik P. G., and Patey G. N., J. Chem. Phys. 88, 7826 (1988). 10.1063/1.454296 - DOI
-
- Torrie G. M. and Patey G. N., J. Chem. Phys. 97, 12909 (1993). 10.1021/j100151a045 - DOI
-
- Kinoshita M. and Harada M., Mol. Phys. 81, 1473 (1994). 10.1080/00268979400101011 - DOI
-
- Kinoshita M., Condens. Matter Phys. 10, 387 (2007).
-
- Kinoshita M. and Hirata F., J. Chem. Phys. 104, 8807 (1996). 10.1063/1.471570 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

