In vitro effects of an in silico-modelled 17β-estradiol derivative in combination with dichloroacetic acid on MCF-7 and MCF-12A cells
- PMID: 21992416
- PMCID: PMC6496326
- DOI: 10.1111/j.1365-2184.2011.00789.x
In vitro effects of an in silico-modelled 17β-estradiol derivative in combination with dichloroacetic acid on MCF-7 and MCF-12A cells
Abstract
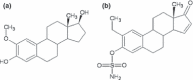
Objectives: To investigate anti-proliferative properties of a novel in silico-modelled 17β-oestradiol derivative (C9), in combination with dichloroacetic acid (DCA), on MCF-7 and MCF-12A cells.
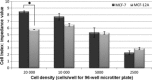
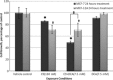
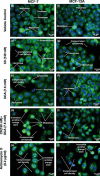
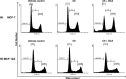
Materials and methods: xCELLigence system was employed to determine optimal seeding number for cells, and crystal violet assay was used to assess cell number and to determine IC(50) value (24 h) for combination treatment. Light and fluorescent microscopy techniques were used to morphologically detect types of cell death. Flow cytometry was used to analyse cell cycle and apoptosis.
Results: Optimal seeding number for 96-well plates was determined to be 5000-10 000 cells/well for both MCF-7 and MCF-12A cells. IC(50) for MCF-7 cells of the combination treatment after 24 h was 130 nm of C9 in conjunction with 7.5 mm of DCA (P < 0.05). In contrast, the same concentration inhibited cell population growth by only 29.3% for MCF-12As after 24-h treatment (P < 0.05). Morphological studies revealed lower cell density of both types of combination-treated cells. Flow cytometric analyses demonstrated increase in sub-G(1) phase in combination-treated MCF-7 cells.
Conclusions: These results demonstrate that the novel 17β-oestradiol derivative C9, in combination with DCA is a potent anti-proliferation treatment, with properties of selectivity towards tumourigenic cells. Thus, this warrants further studies as a potential combination chemotherapeutic agent for further cancer cell lines.
© 2011 Blackwell Publishing Ltd.
Figures




References
-
- Zhou J, Giannakakou P (2005) Targeting microtubules for cancer chemotherapy. Curr. Med. Chem. Anticancer Agents 5, 65–71. - PubMed
-
- Islam MN, Iskander MN (2004) Microtubulin binding sites as target for developing anticancer agents. Mini Rev. Med. Chem. 4, 1077–1104. - PubMed
-
- Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 4, 253–265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

