Expression and reactivation of HIV in a chemokine induced model of HIV latency in primary resting CD4+ T cells
- PMID: 21992606
- PMCID: PMC3215964
- DOI: 10.1186/1742-4690-8-80
Expression and reactivation of HIV in a chemokine induced model of HIV latency in primary resting CD4+ T cells
Abstract
Background: We recently described that HIV latent infection can be established in vitro following incubation of resting CD4+ T-cells with chemokines that bind to CCR7. The main aim of this study was to fully define the post-integration blocks to virus replication in this model of CCL19-induced HIV latency.
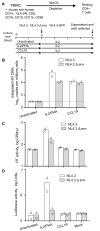
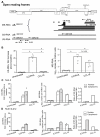
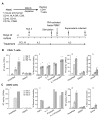
Results: High levels of integrated HIV DNA but low production of reverse transcriptase (RT) was found in CCL19-treated CD4+ T-cells infected with either wild type (WT) NL4.3 or single round envelope deleted NL4.3 pseudotyped virus (NL4.3- Δenv). Supernatants from CCL19-treated cells infected with either WT NL4.3 or NL4.3- Δenv did not induce luciferase expression in TZM-bl cells, and there was no expression of intracellular p24. Following infection of CCL19-treated CD4+ T-cells with NL4.3 with enhanced green fluorescent protein (EGFP) inserted into the nef open reading frame (NL4.3- Δnef-EGFP), there was no EGFP expression detected. These data are consistent with non-productive latent infection of CCL19-treated infected CD4+ T-cells. Treatment of cells with phytohemagluttinin (PHA)/IL-2 or CCL19, prior to infection with WT NL4.3, resulted in a mean fold change in unspliced (US) RNA at day 4 compared to day 0 of 21.2 and 1.1 respectively (p = 0.01; n = 5), and the mean expression of multiply spliced (MS) RNA was 56,000, and 5,000 copies/million cells respectively (p = 0.01; n = 5). In CCL19-treated infected CD4+ T-cells, MS-RNA was detected in the nucleus and not in the cytoplasm; in contrast to PHA/IL-2 activated infected cells where MS RNA was detected in both. Virus could be recovered from CCL19-treated infected CD4+ T-cells following mitogen stimulation (with PHA and phorbyl myristate acetate (PMA)) as well as TNFα, IL-7, prostratin and vorinostat.
Conclusions: In this model of CCL19-induced HIV latency, we demonstrate HIV integration without spontaneous production of infectious virus, detection of MS RNA in the nucleus only, and the induction of virus production with multiple activating stimuli. These data are consistent with ex vivo findings from latently infected CD4+ T-cells from patients on combination antiretroviral therapy, and therefore provide further support of this model as an excellent in vitro model of HIV latency.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

