RAS oncogenes: weaving a tumorigenic web
- PMID: 21993244
- PMCID: PMC3632399
- DOI: 10.1038/nrc3106
RAS oncogenes: weaving a tumorigenic web
Abstract
RAS proteins are essential components of signalling pathways that emanate from cell surface receptors. Oncogenic activation of these proteins owing to missense mutations is frequently detected in several types of cancer. A wealth of biochemical and genetic studies indicates that RAS proteins control a complex molecular circuitry that consists of a wide array of interconnecting pathways. In this Review, we describe how RAS oncogenes exploit their extensive signalling reach to affect multiple cellular processes that drive tumorigenesis.
Conflict of interest statement
Figures
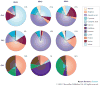

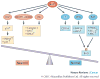
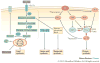

References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Scheidig AJ, Burmester C, Goody RS. The pre-hydrolysis state of p21(ras) in complex with GTP: new insights into the role of water molecules in the GTP hydrolysis reaction of ras-like proteins. Structure. 1999;7:1311–1324. - PubMed
-
- Scheffzek K, et al. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277:333–338. This article described the first three-dimensional structure of the RAS–RASGAP complex, providing insight into the mechanism of GTP hydrolysis and the structural basis for the oncogenicity of RAS mutants. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

