Middle Eocene rodents from Peruvian Amazonia reveal the pattern and timing of caviomorph origins and biogeography
- PMID: 21993503
- PMCID: PMC3282368
- DOI: 10.1098/rspb.2011.1732
Middle Eocene rodents from Peruvian Amazonia reveal the pattern and timing of caviomorph origins and biogeography
Abstract
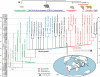
The long-term isolation of South America during most of the Cenozoic produced a highly peculiar terrestrial vertebrate biota, with a wide array of mammal groups, among which caviomorph rodents and platyrrhine primates are Mid-Cenozoic immigrants. In the absence of indisputable pre-Oligocene South American rodents or primates, the mode, timing and biogeography of these extraordinary dispersals remained debated. Here, we describe South America's oldest known rodents, based on a new diverse caviomorph assemblage from the late Middle Eocene (approx. 41 Ma) of Peru, including five small rodents with three stem caviomorphs. Instead of being tied to the Eocene/Oligocene global cooling and drying episode (approx. 34 Ma), as previously considered, the arrival of caviomorphs and their initial radiation in South America probably occurred under much warmer and wetter conditions, around the Mid-Eocene Climatic Optimum. Our phylogenetic results reaffirm the African origin of South American rodents and support a trans-Atlantic dispersal of these mammals during Middle Eocene times. This discovery further extends the gap (approx. 15 Myr) between first appearances of rodents and primates in South America.
Figures


References
-
- Wood A. E., Patterson B. 1959. The rodents of the Deseadan Oligocene of Patagonia and the beginnings of South American rodent evolution. Bull. Mus. Comp. Zool. 120, 281–428
-
- Hoffstetter R. 1972. Origine et dispersion des Rongeurs Hystricognathes. C. R. Acad. Sci. (Paris) 274, 2867–2870
-
- Lavocat R. 1976. Rongeurs Caviomorphes de l'Oligocène de Bolivie: II Rongeurs du bassin Déséadien de Salla-Luribay. Palaeovertebrata 7, 15–90
-
- Patterson B., Wood A. E. 1982. Rodents from the Deseadan Oligocene of Bolivia and the relationship of Caviomorpha. Bull. Mus. Comp. Zool. 149, 372–543
-
- Wyss A. R., Flynn J. J., Norell M. A., Swisher C. C., II, Charrier R., Novacek M. J., McKenna M. C. 1993. South America's earliest rodent and recognition of a new interval of mammalian evolution. Nature 365, 434–43710.1038/365434a0 (doi:10.1038/365434a0) - DOI - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
