Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study
- PMID: 21993710
- PMCID: PMC3210358
- DOI: 10.1007/s00125-011-2325-z
Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study
Abstract
Aims/hypothesis: The renal and cardiovascular protective effects of angiotensin receptor blocker (ARB) remain controversial in type 2 diabetic patients treated with a contemporary regimen including an angiotensin converting enzyme inhibitor (ACEI).
Methods: We examined the effects of olmesartan, an ARB, on primary composite outcome of doubling of serum creatinine, endstage renal disease and death in type 2 diabetic patients with overt nephropathy. Secondary outcome included composite cardiovascular outcomes, changes in renal function and proteinuria. Randomisation and allocation to trial group were carried out by a central computer system. Participants, caregivers, the people carrying out examinations and people assessing the outcomes were blinded to group assignment.
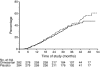
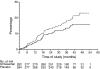
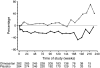
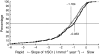
Results: Five hundred and seventy-seven (377 Japanese, 200 Chinese) patients treated with antihypertensive therapy (73.5% [n = 424] received concomitant ACEI), were given either once-daily olmesartan (10-40 mg) (n = 288) or placebo (n = 289) over 3.2 ± 0.6 years (mean±SD). In the olmesartan group, 116 developed the primary outcome (41.1%) compared with 129 (45.4%) in the placebo group (HR 0.97, 95% CI 0.75, 1.24; p = 0.791). Olmesartan significantly decreased blood pressure, proteinuria and rate of change of reciprocal serum creatinine. Cardiovascular death was higher in the olmesartan group than the placebo group (ten vs three cases), whereas major adverse cardiovascular events (cardiovascular death plus non-fatal stroke and myocardial infarction) and all-cause death were similar between the two groups (major adverse cardiovascular events 18 vs 21 cases, all-cause deaths; 19 vs 20 cases). Hyperkalaemia was more frequent in the olmesartan group than the placebo group (9.2% vs 5.3%).
Conclusions/interpretation: Olmesartan was well tolerated but did not improve renal outcome on top of ACEI.
Trial registration: ClinicalTrials.gov NCT00141453.
Figures
References
-
- Ministry of Health, Labor and Welfare (2009) National Health and Nutrition Survey in 2007. www.mhlw.go.jp/houdou/2008/12/h1225-5.html. Accessed on 24 April 2009
-
- Lam TH, Liu LJ, Janus ED, Lam KSL, Hedley AJ. Fibrinogen, other cardiovascular risk factors and diabetes mellitus in Hong Kong Chinese: a community with high prevalence of type 2 diabetes mellitus and impaired glucose tolerance. Diabetic Med. 2000;17:798–806. doi: 10.1046/j.1464-5491.2000.00384.x. - DOI - PubMed
-
- United States Renal Data System (2009) Excerpts from the United States Renal Data System 2008 Annual Data Report: International comparison. Am J Kidney Dis 53(Suppl 1):S297–S308
-
- Japanese Society of Dialysis Therapy: An overview of regular dialysis treatment in Japan as of Dec 31, 2007. http://docs.jsdt.or.jp/overview/. Accessed 24 April 2009
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

