Vascular smooth muscle contractility depends on cell shape
- PMID: 21993765
- PMCID: PMC8388149
- DOI: 10.1039/c1ib00061f
Vascular smooth muscle contractility depends on cell shape
Abstract
The physiologic role of smooth muscle structure in defining arterial function is poorly understood. We aimed to elucidate the relationship between vascular smooth muscle architecture and functional contractile output. Using microcontact printing and muscular thin film technology, we engineered in vitro vascular tissues with strictly defined geometries and tested their contractile function. In all tissues, vascular smooth muscle cells (VSMCs) were highly aligned with in vivo-like spindle architecture, and contracted physiologically in response to stimulation with endothelin-1. However, tissues wherein the VSMCs were forced into exaggerated spindle elongation exerted significantly greater contraction force per unit cross-sectional area than those with smaller aspect ratios. Moreover, this increased contraction did not occur in conjunction with an increase in traditionally measured contractile phenotype markers. These results suggest that cellular architecture within vascular tissues plays a significant role in conferring tissue function and that, in some systems, traditional phenotype characterization is not sufficient to define a functionally contractile population of VSMCs.
Figures

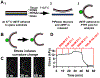


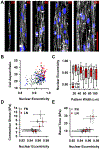
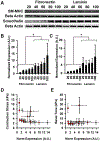
References
-
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428 - PubMed
-
- Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. Faseb J. 2002;16 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

