Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the RESPOND study
- PMID: 21994233
- PMCID: PMC3298666
- DOI: 10.1136/ard.2011.152223
Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the RESPOND study
Abstract
Objective: To compare the efficacy and safety of treatment with infliximab plus methotrexate with methotrexate alone in methotrexate-naive patients with active psoriatic arthritis (PsA).
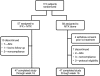
Methods: In this open-label study, patients 18 years and older with active PsA who were naive to methotrexate and not receiving disease-modifying therapy (N=115) were randomly assigned (1:1) to receive either infliximab (5 mg/kg) at weeks 0, 2, 6 and 14 plus methotrexate (15 mg/week); or methotrexate (15 mg/week) alone. The primary assessment was American College of Rheumatology (ACR) 20 response at week 16. Secondary outcome measures included psoriasis area and severity index (PASI), disease activity score in 28 joints (DAS28) and dactylitis and enthesitis assessments.
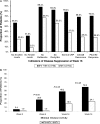
Results: At week 16, 86.3% of patients receiving infliximab plus methotrexate and 66.7% of those receiving methotrexate alone achieved an ACR20 response (p<0.02). Of patients whose baseline PASI was 2.5 or greater, 97.1% receiving infliximab plus methotrexate compared with 54.3% receiving methotrexate alone experienced a 75% or greater improvement in PASI (p<0.0001). Improvements in C-reactive protein levels, DAS28 response and remission rates, dactylitis, fatigue and morning stiffness duration were also significantly greater in the group receiving infliximab. In the infliximab plus methotrexate group, 46% (26/57) had treatment-related adverse events (AE) and two patients had serious AE, compared with 24% with AE (13/54) and no serious AE in the methotrexate-alone group.
Conclusions: Treatment with infliximab plus methotrexate in methotrexate-naive patients with active PsA demonstrated significantly greater ACR20 response rates and PASI75 improvement compared with methotrexate alone and was generally well tolerated. This trial is registered in the US National Institutes of Health clinicaltrials.gov database, identifier NCT00367237.
Conflict of interest statement
Figures

References
-
- Gladman DD. Psoriatic arthritis from Wright's era until today. J Rheumatol Suppl 2009;83:4–8 - PubMed
-
- Koo J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin 1996;14:485–96 - PubMed
-
- Shbeeb M, Uramoto KM, Gibson LE, et al. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982–1991. J Rheumatol 2000;27:1247–50 - PubMed
-
- Alenius GM, Stenberg B, Stenlund H, et al. Inflammatory joint manifestations are prevalent in psoriasis: prevalence study of joint and axial involvement in psoriatic patients, and evaluation of a psoriatic and arthritic questionnaire. J Rheumatol 2002;29:2577–82 - PubMed
-
- Gladman DD, Stafford-Brady F, Chang CH, et al. Longitudinal study of clinical and radiological progression in psoriatic arthritis. J Rheumatol 1990;17:809–12 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

