Biodistribution, toxicology, and radiation dosimetry of 5-HT1A-receptor agonist positron emission tomography ligand [11C]CUMI-101
- PMID: 21994241
- PMCID: PMC4169297
- DOI: 10.1177/1091581811419024
Biodistribution, toxicology, and radiation dosimetry of 5-HT1A-receptor agonist positron emission tomography ligand [11C]CUMI-101
Abstract
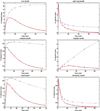
Sprague Dawley rats (10/sex/group) were given a single intravenous (iv) dose of CUMI-101 to determine acute toxicity of CUMI-101 and radiation dosimetry estimations were conducted in baboons with [(11)C]CUMI-101. Intravenous administration of CUMI-101 did not produce overt biologically or toxicologically significant adverse effects except transient hypoactivity immediately after dose in the mid- and high-dose groups, which is not considered to be a dose-limiting toxic effect. No adverse effects were observed in the low-dose group. The no observed adverse effect level (NOAEL) is considered to be 44.05 µg/kg for a single iv dose administration in rats. The maximum tolerated dose (MTD) was estimated to be 881 µg/kg for a single iv dose administration. The Medical Internal Radiation Dose (MIRDOSE) estimates indicate the maximum permissible single-study dosage of [(11)C]CUMI-101 in humans is 52 mCi with testes and urinary bladder as the critical organ for males and females, respectively.
Conflict of interest statement
The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
Figures
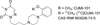
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

