Short-term synaptic plasticity regulates the level of olivocochlear inhibition to auditory hair cells
- PMID: 21994392
- PMCID: PMC3224807
- DOI: 10.1523/JNEUROSCI.6788-10.2011
Short-term synaptic plasticity regulates the level of olivocochlear inhibition to auditory hair cells
Abstract
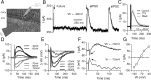
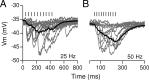
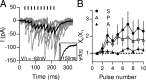
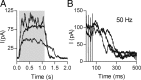
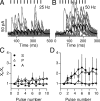
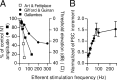
In the mammalian inner ear, the gain control of auditory inputs is exerted by medial olivocochlear (MOC) neurons that innervate cochlear outer hair cells (OHCs). OHCs mechanically amplify the incoming sound waves by virtue of their electromotile properties while the MOC system reduces the gain of auditory inputs by inhibiting OHC function. How this process is orchestrated at the synaptic level remains unknown. In the present study, MOC firing was evoked by electrical stimulation in an isolated mouse cochlear preparation, while OHCs postsynaptic responses were monitored by whole-cell recordings. These recordings confirmed that electrically evoked IPSCs (eIPSCs) are mediated solely by α9α10 nAChRs functionally coupled to calcium-activated SK2 channels. Synaptic release occurred with low probability when MOC-OHC synapses were stimulated at 1 Hz. However, as the stimulation frequency was raised, the reliability of release increased due to presynaptic facilitation. In addition, the relatively slow decay of eIPSCs gave rise to temporal summation at stimulation frequencies >10 Hz. The combined effect of facilitation and summation resulted in a frequency-dependent increase in the average amplitude of inhibitory currents in OHCs. Thus, we have demonstrated that short-term plasticity is responsible for shaping MOC inhibition and, therefore, encodes the transfer function from efferent firing frequency to the gain of the cochlear amplifier.
Figures


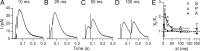



References
-
- Ashmore J. Cochlear outer hair cell motility. Physiol Rev. 2008;88:173–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
