Site-specific proteolytic cleavage of the amino terminus of herpes simplex virus glycoprotein K on virion particles inhibits virus entry
- PMID: 21994443
- PMCID: PMC3233161
- DOI: 10.1128/JVI.06268-11
Site-specific proteolytic cleavage of the amino terminus of herpes simplex virus glycoprotein K on virion particles inhibits virus entry
Abstract
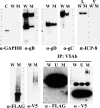
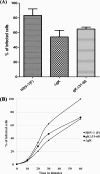
Herpes simplex virus 1 (HSV-1) glycoprotein K (gK) is expressed on virions and functions in entry, inasmuch as HSV-1(KOS) virions devoid of gK enter cells substantially slower than is the case for the parental KOS virus (T. P. Foster, G. V. Rybachuk, and K. G. Kousoulas, J. Virol. 75:12431-12438, 2001). Deletion of the amino-terminal 68-amino-acid (aa) portion of gK caused a reduction in efficiency and kinetics of virus entry similar to that of the gK-null virus in comparison to the HSV-1(F) parental virus. The UL20 membrane protein and gK were readily detected on double-gradient-purified virion preparations. Immuno-electron microscopy confirmed the presence of gK and UL20 on purified virions. Coimmunoprecipitation experiments using purified virions revealed that gK interacted with UL20, as has been shown in virus-infected cells (T. P. Foster, V. N. Chouljenko, and K. G. Kousoulas, J. Virol. 82:6310-6323, 2008). Scanning of the HSV-1(F) viral genome revealed the presence of a single putative tobacco etch virus (TEV) protease site within gD, while additional TEV predicted sites were found within the UL5 (helicase-primase helicase subunit), UL23 (thymidine kinase), UL25 (DNA packaging tegument protein), and UL52 (helicase-primase primase subunit) proteins. The recombinant virus gDΔTEV was engineered to eliminate the single predicted gD TEV protease site without appreciably affecting its replication characteristics. The mutant virus gK-V5-TEV was subsequently constructed by insertion of a gene sequence encoding a V5 epitope tag in frame with the TEV protease site immediately after gK amino acid 68. The gK-V5-TEV, R-gK-V5-TEV (revertant virus), and gDΔTEV viruses exhibited similar plaque morphologies and replication characteristics. Treatment of the gK-V5-TEV virions with TEV protease caused approximately 32 to 34% reduction of virus entry, while treatment of gDΔTEV virions caused slightly increased virus entry. These results provide direct evidence that the gK and UL20 proteins, which are genetically and functionally linked to gB-mediated virus-induced cell fusion, are structural components of virions and function in virus entry. Site-specific cleavage of viral glycoproteins on mature and fully infectious virions utilizing unique protease sites may serve as a generalizable method of uncoupling the roles of viral glycoproteins in virus entry and virion assembly.
Figures






Similar articles
-
Herpes simplex virus 1 protein UL37 interacts with viral glycoprotein gK and membrane protein UL20 and functions in cytoplasmic virion envelopment.J Virol. 2014 Jun;88(11):5927-35. doi: 10.1128/JVI.00278-14. Epub 2014 Mar 5. J Virol. 2014. PMID: 24600000 Free PMC article.
-
The herpes simplex virus type 1 UL20 protein and the amino terminus of glycoprotein K (gK) physically interact with gB.J Virol. 2010 Sep;84(17):8596-606. doi: 10.1128/JVI.00298-10. Epub 2010 Jun 23. J Virol. 2010. PMID: 20573833 Free PMC article.
-
The amino terminus of herpes simplex virus 1 glycoprotein K is required for virion entry via the paired immunoglobulin-like type-2 receptor alpha.J Virol. 2013 Mar;87(6):3305-13. doi: 10.1128/JVI.02982-12. Epub 2013 Jan 9. J Virol. 2013. PMID: 23302878 Free PMC article.
-
Two Sides to Every Story: Herpes Simplex Type-1 Viral Glycoproteins gB, gD, gH/gL, gK, and Cellular Receptors Function as Key Players in Membrane Fusion.Viruses. 2021 Sep 16;13(9):1849. doi: 10.3390/v13091849. Viruses. 2021. PMID: 34578430 Free PMC article. Review.
-
Glycoprotein K of herpes simplex virus: a transmembrane protein encoded by the UL53 gene which regulates membrane fusion.Virus Genes. 1999;18(1):81-90. doi: 10.1023/a:1008025520655. Virus Genes. 1999. PMID: 10334040 Review.
Cited by
-
Binding of HSV-1 glycoprotein K (gK) to signal peptide peptidase (SPP) is required for virus infectivity.PLoS One. 2014 Jan 20;9(1):e85360. doi: 10.1371/journal.pone.0085360. eCollection 2014. PLoS One. 2014. PMID: 24465545 Free PMC article.
-
Phenylalanine residues at the carboxyl terminus of the herpes simplex virus 1 UL20 membrane protein regulate cytoplasmic virion envelopment and infectious virus production.J Virol. 2014 Jul;88(13):7618-27. doi: 10.1128/JVI.00657-14. Epub 2014 Apr 23. J Virol. 2014. PMID: 24760889 Free PMC article.
-
Inhibitors of signal peptide peptidase (SPP) affect HSV-1 infectivity in vitro and in vivo.Exp Eye Res. 2014 Jun;123:8-15. doi: 10.1016/j.exer.2014.04.004. Epub 2014 Apr 24. Exp Eye Res. 2014. PMID: 24768597 Free PMC article.
-
A herpes simplex virus 1 (McKrae) mutant lacking the glycoprotein K gene is unable to infect via neuronal axons and egress from neuronal cell bodies.mBio. 2012 Jul 24;3(4):e00144-12. doi: 10.1128/mBio.00144-12. Print 2012. mBio. 2012. PMID: 22829677 Free PMC article.
-
Alphaherpesvirus glycoprotein E: A review of its interactions with other proteins of the virus and its application in vaccinology.Front Microbiol. 2022 Aug 4;13:970545. doi: 10.3389/fmicb.2022.970545. eCollection 2022. Front Microbiol. 2022. PMID: 35992696 Free PMC article. Review.
References
-
- Arii J., et al. 2010. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature 467:859–862 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

