A virus-like particle-based Epstein-Barr virus vaccine
- PMID: 21994444
- PMCID: PMC3233152
- DOI: 10.1128/JVI.05598-11
A virus-like particle-based Epstein-Barr virus vaccine
Abstract
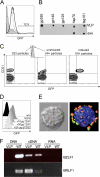
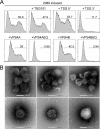
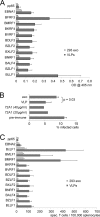
Epstein-Barr Virus (EBV) is an ubiquitous human herpesvirus which can lead to infectious mononucleosis and different cancers. In immunocompromised individuals, this virus is a major cause for morbidity and mortality. Transplant patients who did not encounter EBV prior to immunosuppression frequently develop EBV-associated malignancies, but a prophylactic EBV vaccination might reduce this risk considerably. Virus-like particles (VLPs) mimic the structure of the parental virus but lack the viral genome. Therefore, VLPs are considered safe and efficient vaccine candidates. We engineered a dedicated producer cell line for EBV-derived VLPs. This cell line contains a genetically modified EBV genome which is devoid of all potential viral oncogenes but provides viral proteins essential for the assembly and release of VLPs via the endosomal sorting complex required for transport (ESCRT). Human B cells readily take up EBV-based VLPs and present viral epitopes in association with HLA molecules to T cells. Consequently, EBV-based VLPs are highly immunogenic and elicit humoral and strong CD8+ and CD4+ T cell responses in vitro and in a preclinical murine model in vivo. Our findings suggest that VLP formulations might be attractive candidates to develop a safe and effective polyvalent vaccine against EBV.
Figures


References
-
- Ablashi D. V., et al. 1997. Epstein-Barr virus and Kaposi's sarcoma herpesvirus/human herpesvirus 8, p. In (ed.), IARC monograph on the evaluation of carcinogenic risks to humans. International Association for Research on Cancer, Lyon, France
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

