A virion-associated protein kinase induces apoptosis
- PMID: 21994449
- PMCID: PMC3233169
- DOI: 10.1128/JVI.05294-11
A virion-associated protein kinase induces apoptosis
Abstract
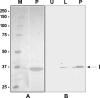
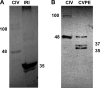
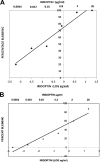
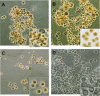
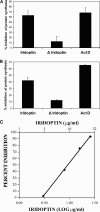
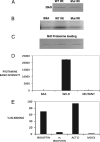
Apoptosis and inhibition of host gene expression are often associated with virus infections. Many viral polypeptides modulate apoptosis by direct interaction with highly conserved apoptotic pathways. Some viruses induce apoptosis during late stages of the infection cycle, while others inhibit apoptosis to facilitate replication or maintain persistent infection. In previous work, we showed that Chilo iridescent virus (CIV) or CIV virion protein extract induces apoptosis in spruce budworm and cotton boll weevil cell cultures. Here, we characterize the product of a CIV gene (iridovirus serine/threonine kinase; istk) with signature sequences for S/T kinase and ATP binding. ISTK appears to belong to the superfamily, vaccinia-related kinases (VRKs). The istk gene was expressed in Pichia pastoris vectors. Purified ISTK (48 kDa) exhibited S/T kinase activity. Treatment with ISTK induced apoptosis in budworm cells. A 35-kDa cleavage product of ISTK retaining key signature sequences was identified during purification. Pichia-expressed 35-kDa polypeptide, designated iridoptin, induced apoptosis and inhibition of host protein synthesis in budworm and boll weevil cells. A mutation in the ATP-binding site eliminated both kinase and apoptosis activity of iridoptin, suggesting that kinase activity is essential for induction of apoptosis. Analysis with custom antibody confirmed that ISTK is a structural component of CIV particles. This is the first demonstration of a viral kinase inducing apoptosis in any virus-host system and the first identification of a factor inducing apoptosis or host protein shutoff for the family Iridoviridae.
Figures


References
-
- Banham A. H., Leader D. P., Smith G. L. 1993. Phosphorylation of ribosomal proteins by the vaccinia virus B1R protein kinase. FEBS Lett. 321:27–31 - PubMed
-
- Bilimoria S. L. March 2001. Use of viral proteins for controlling the cotton boll weevil and other insect pests. U.S. patent 6200561
-
- Bilimoria S. L., Sohi S. S. 1977. Development of an attached strain from a continuous insect cell line. In Vitro 13:461–466 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

