Structure and mutagenesis of the parainfluenza virus 5 hemagglutinin-neuraminidase stalk domain reveals a four-helix bundle and the role of the stalk in fusion promotion
- PMID: 21994464
- PMCID: PMC3233124
- DOI: 10.1128/JVI.06350-11
Structure and mutagenesis of the parainfluenza virus 5 hemagglutinin-neuraminidase stalk domain reveals a four-helix bundle and the role of the stalk in fusion promotion
Abstract
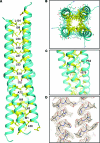
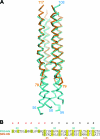
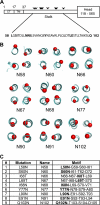
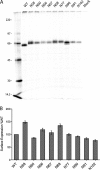
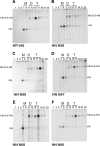
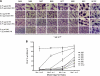
Paramyxovirus entry into cells requires the fusion protein (F) and a receptor binding protein (hemagglutinin-neuraminidase [HN], H, or G). The multifunctional HN protein of some paramyxoviruses, besides functioning as the receptor (sialic acid) binding protein (hemagglutinin activity) and the receptor-destroying protein (neuraminidase activity), enhances F activity, presumably by lowering the activation energy required for F to mediate fusion of viral and cellular membranes. Before or upon receptor binding by the HN globular head, F is believed to interact with the HN stalk. Unfortunately, until recently none of the receptor binding protein crystal structures have shown electron density for the stalk domain. Parainfluenza virus 5 (PIV5) HN exists as a noncovalent dimer-of-dimers on the surface of cells, linked by a single disulfide bond in the stalk. Here we present the crystal structure of the PIV5-HN stalk domain at a resolution of 2.65 Å, revealing a four-helix bundle (4HB) with an upper (N-terminal) straight region and a lower (C-terminal) supercoiled part. The hydrophobic core residues are a mix of an 11-mer repeat and a 3- to 4-heptad repeat. To functionally characterize the role of the HN stalk in F interactions and fusion, we designed mutants along the PIV5-HN stalk that are N-glycosylated to physically disrupt F-HN interactions. By extensive study of receptor binding, neuraminidase activity, oligomerization, and fusion-promoting functions of the mutant proteins, we found a correlation between the position of the N-glycosylation mutants on the stalk structure and their neuraminidase activities as well as their abilities to promote fusion.
Figures



References
-
- Bousse T., Takimoto T., Gorman W. L., Takahashi T., Portner A. 1994. Regions on the hemagglutinin-neuraminidase proteins of human parainfluenza virus type-1 and Sendai virus important for membrane fusion. Virology 204: 506–514 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

