Inhibitory Effects of Quercetin on Muscle-type of Nicotinic Acetylcholine Receptor-Mediated Ion Currents Expressed in Xenopus Oocytes
- PMID: 21994477
- PMCID: PMC3186920
- DOI: 10.4196/kjpp.2011.15.4.195
Inhibitory Effects of Quercetin on Muscle-type of Nicotinic Acetylcholine Receptor-Mediated Ion Currents Expressed in Xenopus Oocytes
Abstract
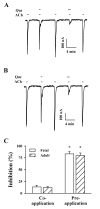
The flavonoid quercetin is a low molecular weight compound generally found in apple, gingko, tomato, onion and other red-colored fruits and vegetables. Like other flavonoids, quercetin has diverse pharmacological actions. However, relatively little is known about the influence of quercetin effects in the regulation of ligand-gated ion channels. Previously, we reported that quercetin regulates subsets of nicotinic acetylcholine receptors such as α3β4, α7 and α9α10. Presently, we investigated the effects of quercetin on muscle-type of nicotinic acetylcholine receptor channel activity expressed in Xenopus oocytes after injection of cRNA encoding human fetal or adult muscle-type of nicotinic acetylcholine receptor subunits. Acetylcholine treatment elicited an inward peak current (I(ACh)) in oocytes expressing both muscle-type of nicotinic acetylcholine receptors and co-treatment of quercetin with acetylcholine inhibited I(ACh). Pre-treatment of quercetin further inhibited I(ACh) in oocytes expressing adult and fetal muscle-type nicotinic acetylcholine receptors. The inhibition of I(ACh) by quercetin was reversible and concentration-dependent. The IC(50) of quercetin was 18.9±1.2 µM in oocytes expressing adult muscle-type nicotinic acetylcholine receptor. The inhibition of I(ACh) by quercetin was voltage-independent and non-competitive. These results indicate that quercetin might regulate human muscle-type nicotinic acetylcholine receptor channel activity and that quercetin-mediated regulation of muscle-type nicotinic acetylcholine receptor might be coupled to regulation of neuromuscular junction activity.
Keywords: Flavonoids; Muscle-type nicotinic acetylcholine receptors; Quercetin; Xenopus oocyte.
Figures



References
-
- Jensen ML, Schousboe A, Ahring PK. Charge selectivity of the Cys-loop family of ligand-gated ion channels. J Neurochem. 2005;92:217–225. - PubMed
-
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. - PubMed
-
- Boulter J, Evans K, Goldman D, Martin G, Treco D, Heinemann S, Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. Nature. 1986;319:368–374. - PubMed
-
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3:102–114. - PubMed

