Viroid pathogenicity: one process, many faces
- PMID: 21994551
- PMCID: PMC3185495
- DOI: 10.3390/v1020298
Viroid pathogenicity: one process, many faces
Abstract
Despite the non-coding nature of their small RNA genomes, the visible symptoms of viroid infection resemble those associated with many plant virus diseases. Recent evidence indicates that viroid-derived small RNAs acting through host RNA silencing pathways play a key role in viroid pathogenicity. Host responses to viroid infection are complex, involving signaling cascades containing host-encoded protein kinases and crosstalk between hormonal and defense-signaling pathways. Studies of viroid-host interaction in the context of entire biochemical or developmental pathways are just beginning, and many working hypotheses have yet to be critically tested.
Keywords: RNA silencing; disease induction; viroid pathogenicity.
Figures
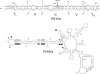

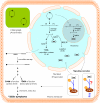
References
-
- Diener TO. Potato spindle tuber “virus” IV. A replicating, low molecular weight RNA. Virology. 1971;45:411–428. - PubMed
-
- Gross HJ, Domdey H, Lossow C, Jank P, Raba M, Alberty H, Sänger H-L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978;273:203–208. - PubMed
-
- Dickson E, Robertson HD, Niblett CL, Horst RK, Zaitlin M. Minor differences between nucleotide sequences of mild and severe strains of potato spindle tuber viroid. Nature. 1979;277:60–62.
LinkOut - more resources
Full Text Sources

