Development of ST-246® for Treatment of Poxvirus Infections
- PMID: 21994624
- PMCID: PMC3185582
- DOI: 10.3390/v2112409
Development of ST-246® for Treatment of Poxvirus Infections
Abstract
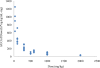
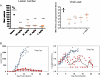
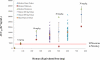
ST-246 (Tecovirimat) is a small synthetic antiviral compound being developed to treat pathogenic orthopoxvirus infections of humans. The compound was discovered as part of a high throughput screen designed to identify inhibitors of vaccinia virus-induced cytopathic effects. The antiviral activity is specific for orthopoxviruses and the compound does not inhibit the replication of other RNA- and DNA-containing viruses or inhibit cell proliferation at concentrations of compound that are antiviral. ST-246 targets vaccinia virus p37, a viral protein required for envelopment and secretion of extracellular forms of virus. The compound is orally bioavailable and protects multiple animal species from lethal orthopoxvirus challenge. Preclinical safety pharmacology studies in mice and non-human primates indicate that ST-246 is readily absorbed by the oral route and well tolerated with the no observable adverse effect level (NOAEL) in mice measured at 2000 mg/kg and the no observable effect level (NOEL) in non-human primates measured at 300 mg/kg. Drug substance and drug product processes have been developed and commercial scale batches have been produced using Good Manufacturing Processes (GMP). Human phase I clinical trials have shown that ST-246 is safe and well tolerated in healthy human volunteers. Based on the results of the clinical evaluation, once a day dosing should provide plasma drug exposure in the range predicted to be antiviral based on data from efficacy studies in animal models of orthopoxvirus disease. These data support the use of ST-246 as a therapeutic to treat pathogenic orthopoxvirus infections of humans.
Keywords: ST-246; Smallpox; Tecovirimat; antiviral drug; egress inhibitor; orthopoxvirus; p37.
Figures



References
-
- Fenner F, Henderson DA, Arita I, Jazek Z, Ladnyi ID. Smallpox and Its Eradication. World Health Organization; Geneva, Switzerland: 1988. pp. 1–1421.
-
- Parker S, Nuara A, Buller RM, Schultz DA. Human monkeypox: An emerging zoonotic disease. Future Microbiol. 2007;2:17–34. - PubMed
-
- Monath TP, Caldwell JR, Mundt W, Fusco J, Johnson CS, Buller M, Liu J, Gardner B, Downing G, Blum PS, Kemp T, Nichols R, Weltzin R. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—A second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31–S44. - PMC - PubMed
-
- Baxby D, Bennett M, Getty B. Human cowpox 1969–93: A review based on 54 cases. Br J Dermatol. 1994;131:598–607. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

