West Nile virus: immunity and pathogenesis
- PMID: 21994755
- PMCID: PMC3185772
- DOI: 10.3390/v3060811
West Nile virus: immunity and pathogenesis
Abstract
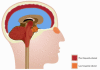
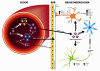
West Nile virus (WNV) is a neurotropic, arthropod-borne flavivirus that is maintained in an enzootic cycle between mosquitoes and birds, but can also infect and cause disease in horses and humans. WNV is endemic in parts of Africa, Europe, the Middle East, and Asia, and since 1999 has spread to North America, Mexico, South America, and the Caribbean. WNV infects the central nervous system (CNS) and can cause severe disease in a small minority of infected humans, mostly immunocompromised or the elderly. This review discusses some of the mechanisms by which the immune system can limit dissemination of WNV infection and elaborates on the mechanisms involved in pathogenesis. Reasons for susceptibility to WNV-associated neuroinvasive disease in less than 1% of cases remain unexplained, but one favored hypothesis is that the involvement of the CNS is associated with a weak immune response allowing robust WNV replication in the periphery and spread of the virus to the CNS.
Keywords: West Nile virus; central nervous system; neuroinvasion; pathogenesis.
Figures
References
-
- Dauphin G, Zientara S, Zeller H, Murgue B. West Nile: Worldwide current situation in animals and humans. Comp. Immunol. Microbiol. Infect. Dis. 2004;27:343–355. - PubMed
-
- Komar N, Clark GG. West Nile virus activity in Latin America and the Caribbean. Rev. Panam. Salud Public. 2006;19:112–117. - PubMed
-
- Lanciotti RS, Ebel GD, Deubel V, Kerst AJ, Murri S, Meyer R, Bowen M, McKinney N, Morrill WE, Crabtree MB, et al. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology. 2002;298:96–105. - PubMed
-
- Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, et al. The outbreak of West Nile virus infection in the New York City area in 1999. New Engl. J. Med. 2001;344:1807–1814. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

