C7a, a biphosphinic cyclopalladated compound, efficiently controls the development of a patient-derived xenograft model of adult T cell leukemia/lymphoma
- PMID: 21994769
- PMCID: PMC3185797
- DOI: 10.3390/v3071041
C7a, a biphosphinic cyclopalladated compound, efficiently controls the development of a patient-derived xenograft model of adult T cell leukemia/lymphoma
Abstract
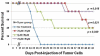
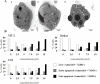
Adult T-cell leukemia/lymphoma (ATLL) is a highly aggressive disease that occurs in individuals infected with the human T lymphotropic virus type 1 (HTLV-1). Patients with aggressive ATLL have a poor prognosis because the leukemic cells are resistant to conventional chemotherapy. We have investigated the therapeutic efficacy of a biphosphinic cyclopalladated complex {Pd(2) [S(-)C(2), N-dmpa](2) (μ-dppe)Cl(2)}, termed C7a, in a patient-derived xenograft model of ATLL, and investigated the mechanism of C7a action in HTLV-1-positive and negative transformed T cell lines in vitro. In vivo survival studies in immunocompromised mice inoculated with human RV-ATL cells and intraperitoneally treated with C7a led to significantly increased survival of the treated mice. We investigated the mechanism of C7a activity in vitro and found that it induced mitochondrial release of cytochrome c, caspase activation, nuclear condensation and DNA degradation. These results suggest that C7a triggers apoptotic cell death in both HTLV-1 infected and uninfected human transformed T-cell lines. Significantly, C7a was not cytotoxic to peripheral blood mononuclear cells (PBMC) from healthy donors and HTLV-1-infected individuals. C7a inhibited more than 60% of the ex vivo spontaneous proliferation of PBMC from HTLV-1-infected individuals. These results support a potential therapeutic role for C7a in both ATLL and HTLV-1-negative T-cell lymphomas.
Keywords: ATLL; HTLV-1; apoptosis; chemotherapy; cyclopalladated compound; xenograft model.
Figures




References
-
- Feuer G, Zack JA, Harrington WJ, Jr, Valderama R, Rosenblatt JD, Wachsman W, Baird SM, Chen IS. Establishment of HTLV- I T-cell lymphomas in severe combined immunodeficient mice. Blood. 1993;82:722–731. - PubMed
-
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005;174:6477–6489. - PubMed
-
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007;7:118–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

