The adaptor proteins p140CAP and p130CAS as molecular hubs in cell migration and invasion of cancer cells
- PMID: 21994904
- PMCID: PMC3189826
The adaptor proteins p140CAP and p130CAS as molecular hubs in cell migration and invasion of cancer cells
Abstract
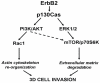
The assembly of molecular hubs upon integrin and growth factor stimulation represents a preferential way to transduce signals throughout the cell. Among the intracellular kinases that are responsive to integrin and growth factor activation, Src Family Kinases (SFKs) are crucial regulators of cell migration and invasion. Increasing evidence highlight the importance of adaptor proteins in these processes, based on their ability to create signalling platforms that control downstream signals. Among these adaptors we will discuss the molecular features of p130Cas and p140Cap proteins in terms of regulation of cell migration and invasion in normal and transformed cells.
Keywords: integrins; invasion; migration; p130Cas; p140Cap; receptor tyrosine kinases.
Figures




References
-
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. - PubMed
-
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. - PubMed
-
- Berx G, Raspe E, Christofori G, Thiery JP, Sleeman JP. Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer. Clin Exp Metastasis. 2007;24:587–597. - PubMed
-
- Giancotti FG. A structural view of integrin activation and signaling. Dev Cell. 2003;4:149–151. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
