Lactose permease and the alternating access mechanism
- PMID: 21995338
- PMCID: PMC3210931
- DOI: 10.1021/bi2014294
Lactose permease and the alternating access mechanism
Abstract
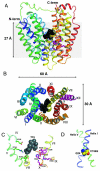
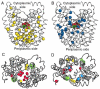
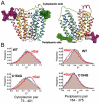
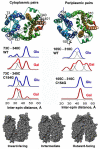
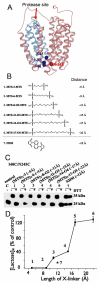
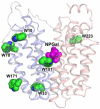
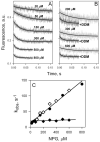
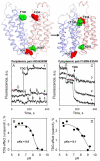
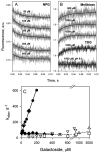
Crystal structures of the lactose permease of Escherichia coli (LacY) reveal 12, mostly irregular transmembrane α-helices surrounding a large cavity open to the cytoplasm and a tightly sealed periplasmic side (inward-facing conformation) with the sugar-binding site at the apex of the cavity and inaccessible from the periplasm. However, LacY is highly dynamic, and binding of a galactopyranoside causes closing of the inward-facing cavity with opening of a complementary outward-facing cavity. Therefore, the coupled, electrogenic translocation of a sugar and a proton across the cytoplasmic membrane via LacY very likely involves a global conformational change that allows alternating access of sugar- and H(+)-binding sites to either side of the membrane. Here the various biochemical and biophysical approaches that provide strong support for the alternating access mechanism are reviewed. Evidence is also presented indicating that opening of the periplasmic cavity is probably the limiting step for binding and perhaps transport.
Figures
Similar articles
-
Probing the periplasmic-open state of lactose permease in response to sugar binding and proton translocation.J Mol Biol. 2010 Dec 3;404(3):506-21. doi: 10.1016/j.jmb.2010.09.045. Epub 2010 Sep 25. J Mol Biol. 2010. PMID: 20875429 Free PMC article.
-
The alternating access transport mechanism in LacY.J Membr Biol. 2011 Jan;239(1-2):85-93. doi: 10.1007/s00232-010-9327-5. Epub 2010 Dec 16. J Membr Biol. 2011. PMID: 21161516 Free PMC article. Review.
-
Residues gating the periplasmic pathway of LacY.J Mol Biol. 2009 Nov 27;394(2):219-25. doi: 10.1016/j.jmb.2009.09.043. Epub 2009 Sep 23. J Mol Biol. 2009. PMID: 19781551 Free PMC article.
-
Sugar binding induces the same global conformational change in purified LacY as in the native bacterial membrane.Proc Natl Acad Sci U S A. 2010 May 25;107(21):9903-8. doi: 10.1073/pnas.1004515107. Epub 2010 May 10. Proc Natl Acad Sci U S A. 2010. PMID: 20457922 Free PMC article.
-
Lessons from lactose permease.Annu Rev Biophys Biomol Struct. 2006;35:67-91. doi: 10.1146/annurev.biophys.35.040405.102005. Annu Rev Biophys Biomol Struct. 2006. PMID: 16689628 Free PMC article. Review.
Cited by
-
Mechanism of coupling drug transport reactions located in two different membranes.Front Microbiol. 2015 Feb 24;6:100. doi: 10.3389/fmicb.2015.00100. eCollection 2015. Front Microbiol. 2015. PMID: 25759685 Free PMC article. Review.
-
Fluorescence resonance energy transfer (FRET) and proximity ligation assays reveal functionally relevant homo- and heteromeric complexes among hyaluronan synthases HAS1, HAS2, and HAS3.J Biol Chem. 2015 May 1;290(18):11479-90. doi: 10.1074/jbc.M115.640581. Epub 2015 Mar 20. J Biol Chem. 2015. PMID: 25795779 Free PMC article.
-
A Numbering System for MFS Transporter Proteins.Front Mol Biosci. 2016 Jun 2;3:21. doi: 10.3389/fmolb.2016.00021. eCollection 2016. Front Mol Biosci. 2016. PMID: 27314000 Free PMC article.
-
Structure and mechanism of membrane transporters.Sci Rep. 2022 Aug 2;12(1):13248. doi: 10.1038/s41598-022-17524-1. Sci Rep. 2022. PMID: 35918457 Free PMC article.
-
Galactoside-binding site in LacY.Biochemistry. 2014 Mar 11;53(9):1536-43. doi: 10.1021/bi401716z. Epub 2014 Feb 26. Biochemistry. 2014. PMID: 24520888 Free PMC article.
References
-
- Saier MH., Jr. Families of transmembrane sugar transport proteins. Mol Microbiol. 2000;35:699–710. - PubMed
-
- West IC. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970;41:655–661. - PubMed
-
- Kaback HR, Sahin-Toth M, Weinglass AB. The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol. 2001;2:610–620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

