NSAID-based γ-secretase modulators do not bind to the amyloid-β polypeptide
- PMID: 21995415
- PMCID: PMC3272834
- DOI: 10.1021/bi201371j
NSAID-based γ-secretase modulators do not bind to the amyloid-β polypeptide
Abstract
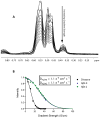
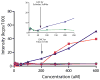
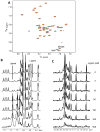
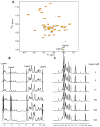
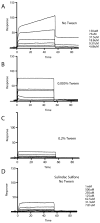
γ-Secretase modulators (GSMs) have received much attention as potential therapeutic agents for Alzheimer's disease (AD). GSMs increase the ratio between short and long forms of the amyloid-β (Aβ) polypeptides produced by γ-secretase and thereby decrease the amount of the toxic amyloid species. However, the mechanism of action of these agents is still poorly understood. One recent paper [Richter et al. (2010) Proc. Natl. Acad. Sci. U. S. A.107, 14597-14602] presented data that were interpreted to support direct binding of the GSM sulindac sulfide to Aβ(42), supporting the notion that GSM action is linked to direct binding of these compounds to the Aβ domain of its immediate precursor, the 99-residue C-terminal domain of the amyloid precursor protein (C99, also known as the β-CTF). Here, contrasting results are presented that indicate there is no interaction between monomeric sulindac sulfide and monomeric forms of Aβ42. Instead, it was observed that sulindac sulfide is itself prone to form aggregates that can bind nonspecifically to Aβ42 and trigger its aggregation. This observation, combined with data from previous work [Beel et al. (2009) Biochemistry48, 11837-11839], suggests both that the poor behavior of some NSAID-based GSMs in solution may obscure results of binding assays and that NSAID-based GSMs do not function by directly targeting C99. It was also observed that another GSM, flurbiprofen, fails to bind to monomeric Aβ42 or to C99 reconstituted into bilayered lipid vesicles. These results disfavor the hypothesis that these NSAID-based GSMs exert their modulatory effect by directly targeting a site located in the Aβ42 domain of free C99.
Figures
References
-
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. - PubMed
-
- Oehlrich D, Berthelot DJ, Gijsen HJ. Gamma-Secretase Modulators as Potential Disease Modifying Anti-Alzheimer's Drugs. J Med Chem 2010 - PubMed
-
- Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004;62:1984–1989. - PubMed
-
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
-
- Glenner GG, Wong CW, Quaranta V, Eanes ED. The amyloid deposits in Alzheimer's disease: their nature and pathogenesis. Appl Pathol. 1984;2:357–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

