Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide
- PMID: 21995440
- PMCID: PMC3207972
- DOI: 10.1186/1742-2094-8-139
Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide
Abstract
Background: Brain microvascular pericytes are important constituents of the neurovascular unit. These cells are physically the closest cells to the microvascular endothelial cells in brain capillaries. They significantly contribute to the induction and maintenance of the barrier functions of the blood-brain barrier. However, very little is known about their immune activities or their roles in neuroinflammation. Here, we focused on the immunological profile of brain pericytes in culture in the quiescent and immune-challenged state by studying their production of immune mediators such as nitric oxide (NO), cytokines, and chemokines. We also examined the effects of immune challenge on pericyte expression of low density lipoprotein receptor-related protein-1 (LRP-1), a protein involved in the processing of amyloid precursor protein and the brain-to-blood efflux of amyloid-β peptide.
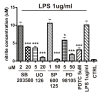
Methods: Supernatants were collected from primary cultures of mouse brain pericytes. Release of nitric oxide (NO) was measured by the Griess reaction and the level of S-nitrosylation of pericyte proteins measured with a modified "biotin-switch" method. Specific mitogen-activated protein kinase (MAPK) pathway inhibitors were used to determine involvement of these pathways on NO production. Cytokines and chemokines were analyzed by multianalyte technology. The expression of both subunits of LRP-1 was analyzed by western blot.
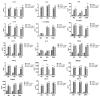
Results: Lipopolysaccharide (LPS) induced release of NO by pericytes in a dose-dependent manner that was mediated through MAPK pathways. Nitrative stress resulted in S-nitrosylation of cellular proteins. Eighteen of twenty-three cytokines measured were released constitutively by pericytes or with stimulation by LPS, including interleukin (IL)-12, IL-13, IL-9, IL-10, granulocyte-colony stimulating factor, granulocyte macrophage-colony stimulating factor, eotaxin, chemokine (C-C motif) ligand (CCL)-3, and CCL-4. Pericyte expressions of both subunits of LRP-1 were upregulated by LPS.
Conclusions: Our results show that cultured mouse brain microvascular pericytes secrete cytokines, chemokines, and nitric oxide and respond to the innate immune system stimulator LPS. These immune properties of pericytes are likely important in their communication within the neurovascular unit and provide a mechanism by which they participate in neuroinflammatory processes in brain infections and neurodegenerative diseases.
Figures

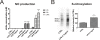

Similar articles
-
Brain pericytes among cells constituting the blood-brain barrier are highly sensitive to tumor necrosis factor-α, releasing matrix metalloproteinase-9 and migrating in vitro.J Neuroinflammation. 2011 Aug 26;8:106. doi: 10.1186/1742-2094-8-106. J Neuroinflammation. 2011. PMID: 21867555 Free PMC article.
-
Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro.Brain Res. 2014 Mar 6;1550:1-8. doi: 10.1016/j.brainres.2014.01.004. Epub 2014 Jan 10. Brain Res. 2014. PMID: 24418464
-
Tumor necrosis factor-α-stimulated brain pericytes possess a unique cytokine and chemokine release profile and enhance microglial activation.Neurosci Lett. 2014 Aug 22;578:133-8. doi: 10.1016/j.neulet.2014.06.052. Epub 2014 Jun 30. Neurosci Lett. 2014. PMID: 24993300
-
Beyond barrier functions: Roles of pericytes in homeostasis and regulation of neuroinflammation.J Neurosci Res. 2020 Dec;98(12):2390-2405. doi: 10.1002/jnr.24715. Epub 2020 Aug 20. J Neurosci Res. 2020. PMID: 32815569 Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
1,25-Dihydroxyvitamin D3 attenuates endotoxin-induced production of inflammatory mediators by inhibiting MAPK activation in primary cortical neuron-glia cultures.J Neuroinflammation. 2015 Aug 12;12:147. doi: 10.1186/s12974-015-0370-0. J Neuroinflammation. 2015. PMID: 26259787 Free PMC article.
-
Cultured pericytes from human brain show phenotypic and functional differences associated with differential CD90 expression.Sci Rep. 2016 May 24;6:26587. doi: 10.1038/srep26587. Sci Rep. 2016. PMID: 27215737 Free PMC article.
-
Aflatoxin B1 Exposure Aggravates Neurobehavioral Deficits and Immune Dysfunctions of Th1, Th9, Th17, Th22, and T Regulatory Cell-Related Transcription Factor Signaling in the BTBR T+Itpr3tf/J Mouse Model of Autism.Brain Sci. 2023 Oct 27;13(11):1519. doi: 10.3390/brainsci13111519. Brain Sci. 2023. PMID: 38002479 Free PMC article.
-
Study protocol: understanding the pathophysiologic mechanisms underlying delirium in older people undergoing hip fracture surgery.BMC Geriatr. 2021 Nov 4;21(1):633. doi: 10.1186/s12877-021-02584-1. BMC Geriatr. 2021. PMID: 34736422 Free PMC article.
-
The fibrotic scar in neurological disorders.Brain Pathol. 2014 Jul;24(4):404-13. doi: 10.1111/bpa.12162. Brain Pathol. 2014. PMID: 24946078 Free PMC article.
References
-
- Mae M, Armulik A, Betsholt C. Getting to Know the Cast-Cellular Interactions and Signaling at the Neurovascular Unit. Curr Pharm Des. 2011;9:9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

