Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen
- PMID: 21995590
- PMCID: PMC3233274
- DOI: 10.3109/13625187.2011.614029
Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen
Abstract
Objectives: The primary objective was to assess the efficacy, cycle control and tolerability of a monophasic combined oral contraceptive (COC) containing nomegestrol acetate (NOMAC) and 17β-oestradiol (E2). Effects on acne were evaluated as a secondary objective. Results were compared to those of a COC containing drospirenone (DRSP) and ethinylestradiol (EE).
Methods: Women (aged 18-50 years) were randomised to receive NOMAC/E2 (2.5 mg/1.5 mg) in a 24/4-day regimen (n=1591) or DRSP/EE (3 mg/30 μg) in a 21/7-day regimen (n=535) for 13 cycles.
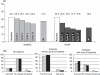
Results: Estimated Pearl Indices for NOMAC/E2 and DRSP/EE were 0.38 and 0.81 in women aged≤35 years and 0.31 and 0.66 for all women (18-50 years), respectively. Scheduled withdrawal bleedings were shorter and lighter among users of NOMAC/E2 and were sometimes absent altogether. Intracyclic bleeding/spotting was infrequent in both groups, and decreased over time. Type and frequency of adverse events were similar to those typically reported for COCs.
Conclusions: These data show that NOMAC/E2 provides high contraceptive efficacy with acceptable cycle control as well as an overall adverse event profile similar to that of DRSP/EE.
Figures
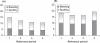
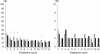
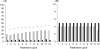
References
-
- UN Department of Economic and Social Affairs Population Division. World Contraceptive Use. Accessed 11 June 2010 from: http://www.un.org/esa/population/publications/contraceptive2009/contrace....
-
- Rosenberg MJ, Meyers A, Roy V. Efficacy cycle control, and side effects of low- and lower-dose oral contraceptives: A randomized trial of 20 micrograms and 35 micrograms estrogen preparations. Contraception. 999;60:321–9. - PubMed
-
- Dinger JC, Heinemann LA, Kuhl-Habich D. The safety of a drospirenone-containing oral contraceptive: Final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344–54. - PubMed
-
- Cerel-Suhl SL, Yeager BF. Update on oral contraceptive pills. Am Fam Physician. 1999;60:2073–84. - PubMed
-
- Sitruk-Ware R. New progestagens for contraceptive use. Hum Reprod Update. 2006;12:169–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
