Distinct levels of Sox9 expression mark colon epithelial stem cells that form colonoids in culture
- PMID: 21995959
- PMCID: PMC3345960
- DOI: 10.1152/ajpgi.00277.2011
Distinct levels of Sox9 expression mark colon epithelial stem cells that form colonoids in culture
Abstract
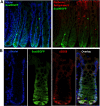
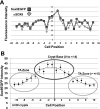
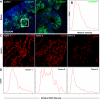
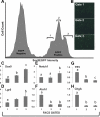
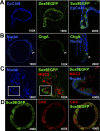
Sox9 is an high-mobility group box transcription factor that is expressed in the stem cell zone of the small intestine and colon. We have previously used a Sox9EGFP mouse model to demonstrate that discrete levels of Sox9 expression mark small intestine epithelial stem cells that form crypt/villus-like structures in a three-dimensional culture system (Formeister EJ, Sionas AL, Lorance DK, Barkley CL, Lee GH, Magness ST. Am J Physiol Gastrointest Liver Physiol 296: G1108-G1118, 2009; Gracz AD, Ramalingam S, Magness ST. Am J Physiol Gastrointest Liver Physiol 298: G590-G600, 2010). In the present study, we hypothesized that discrete levels of Sox9 expression would also mark colonic epithelial stem cells (CESCs). Using the Sox9EGFP mouse model, we show that lower levels of Sox9 mark cells in the transit-amplifying progenitor cell zone, while higher levels of Sox9 mark cells in the colonic crypt base. Furthermore, we demonstrate that variable SOX9 levels persist in cells of colonic adenomas from mice and humans. Cells expressing lower Sox9 levels demonstrate gene expression profiles consistent with more differentiated populations, and cells expressing higher Sox9 levels are consistent with less differentiated populations. When placed in culture, cells expressing the highest levels of Sox9 formed "colonoids," which are defined as bodies of cultured colonic epithelial cells that possess multiple cryptlike structures and a pseudolumen. Cells expressing the highest levels of Sox9 also demonstrate multipotency and self-renewal in vitro, indicating functional stemness. These data suggest a dose-dependent role for Sox9 in normal CESCs and cells comprising colon tumors. Furthermore, distinct Sox9 levels represent a new biomarker to study CESC and progenitor biology in physiological and disease states.
Figures


References
-
- Abdel-Samad R, Zalzali H, Rammah C, Giraud J, Naudin C, Dupasquier S, Poulat F, Boizet-Bonhoure B, Lumbroso S, Mouzat K, Bonnans C, Pignodel C, Raynaud P, Fort P, Quittau-Prevostel C, Blache P. MiniSOX9, a dominant-negative variant in colon cancer cells. Oncogene 30: 2493–2503, 2011 - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007 - PubMed
-
- Cai WB, Roberts SA, Potten CS. The number of clonogenic cells in crypts in three regions of murine large intestine. Int J Radiat Biol 71: 573–579, 1997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

