A phase II evaluation of trabectedin in the treatment of advanced, persistent, or recurrent uterine leiomyosarcoma: a gynecologic oncology group study
- PMID: 21996263
- PMCID: PMC4497524
- DOI: 10.1016/j.ygyno.2011.09.019
A phase II evaluation of trabectedin in the treatment of advanced, persistent, or recurrent uterine leiomyosarcoma: a gynecologic oncology group study
Abstract
Objective: To estimate activity and safety of trabectedin 1.5 mg/m2 IV over 24 hours every 3 weeks (1 cycle) in uterine leiomyosarcoma.
Methods: Patients with chemotherapy naive, advanced, persistent or recurrent uterine leiomyosarcoma, acceptable organ function and PS≤2 were eligible. A two-stage design was utilized. Three responses were required in the first stage to initiate the second stage; the target sample size was 40 for the combined stages. If the true response rate was 10%, the study design provided a 95% chance of correctly classifying the treatment as "inactive." Conversely, if the true response rate was 30%, then the average probability of correctly classifying the treatment as active would be 90%.
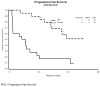
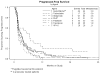
Results: Twenty patients were eligible and evaluable. The median number of cycles was 10 (123 total cycles, range 2-29). The number of patients with partial responses was 2 (10%; 95% confidence interval of 1.2%-31.7%). Response durations were 3.3 and 5.7 months. Ten patients had stable disease (50%). The median progression-free survival (PFS) and overall survival were 5.8 months and greater than 26.1 months (median not reached), respectively. Observed grade 3/4 toxicity included: neutropenia 16/20 (1 infection); thrombocytopenia 3/20; metabolic 3/20; anemia, gastrointestinal and vascular 1/20 each. There were no treatment related deaths nor cases of liver failure.
Conclusions: Although a second stage of accrual was not indicated based on the overall response rate, the drug was well tolerated.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. Bradley Monk has an honoraria and research funding from Johnson & Johnson. All other co-authors have no conflicts of interest to declare.
Figures
Similar articles
-
Efficacy and safety of trabectedin or dacarbazine in patients with advanced uterine leiomyosarcoma after failure of anthracycline-based chemotherapy: Subgroup analysis of a phase 3, randomized clinical trial.Gynecol Oncol. 2017 Sep;146(3):531-537. doi: 10.1016/j.ygyno.2017.06.018. Epub 2017 Jun 24. Gynecol Oncol. 2017. PMID: 28651804 Free PMC article. Clinical Trial.
-
Efficacy of trabectedin in advanced soft tissue sarcoma: beyond lipo- and leiomyosarcoma.Drug Des Devel Ther. 2015 Oct 27;9:5785-91. doi: 10.2147/DDDT.S92395. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26604682 Free PMC article.
-
Activity of trabectedin (ET-743, Yondelis) in metastatic uterine leiomyosarcoma.Gynecol Oncol. 2006 Sep;102(3):421-4. doi: 10.1016/j.ygyno.2006.04.025. Epub 2006 Jun 23. Gynecol Oncol. 2006. PMID: 16797679
-
An increasing role for trabectedin in gynecological cancers: efficacy in uterine sarcomas.Int J Gynecol Cancer. 2011 May;21 Suppl 1:S3-5. doi: 10.1097/IGC.0b013e318217b34d. Int J Gynecol Cancer. 2011. PMID: 21540667 Review.
-
The safety and efficacy of trabectedin for the treatment of liposarcoma or leiomyosarcoma.Expert Rev Anticancer Ther. 2016 May;16(5):473-84. doi: 10.1080/14737140.2016.1174582. Epub 2016 Apr 22. Expert Rev Anticancer Ther. 2016. PMID: 27043847 Review.
Cited by
-
A review of treatment of uterine leiomyosarcomas.Curr Oncol Rep. 2013 Dec;15(6):581-7. doi: 10.1007/s11912-013-0350-4. Curr Oncol Rep. 2013. PMID: 24136566 Review.
-
Trabectedin, a drug acting on both cancer cells and the tumour microenvironment.Br J Cancer. 2014 Aug 12;111(4):646-50. doi: 10.1038/bjc.2014.149. Epub 2014 Apr 22. Br J Cancer. 2014. PMID: 24755886 Free PMC article. Review.
-
The role of adjuvant therapy in uterine leiomyosarcoma.Expert Rev Anticancer Ther. 2016;16(1):45-55. doi: 10.1586/14737140.2016.1115724. Epub 2015 Nov 26. Expert Rev Anticancer Ther. 2016. PMID: 26558647 Free PMC article. Review.
-
Trabectedin in Advanced High-Grade Uterine Leiomyosarcoma: A Case Report Illustrating the Value of (18)FDG-PET-CT in Assessing Treatment Response.Case Rep Oncol. 2014 Feb 27;7(1):132-8. doi: 10.1159/000355224. eCollection 2014 Jan. Case Rep Oncol. 2014. PMID: 24707261 Free PMC article.
-
Long-Lasting Response to Trabectedin in a Patient with Metastatic Uterine Leiomyosarcoma: A Case Report.Case Rep Oncol. 2018 Feb 9;11(1):81-89. doi: 10.1159/000486638. eCollection 2018 Jan-Apr. Case Rep Oncol. 2018. PMID: 29515415 Free PMC article.
References
-
- D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116:131–9. - PubMed
-
- Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. Prognostic factors in early stage uterine sarcoma. Cancer. 1993;71:1702–9. - PubMed
-
- Kanjeekal S, Chambers A, Fung MF, Verma S. Systemic therapy for advanced uterine sarcoma: a systematic review of the literature. Gynecol Oncol. 2005;97:624–37. - PubMed
-
- Mahdavi A, Monk BJ, Ragazzo J, Hunter MI, Lentz SE, Vasilev SA, et al. Pelvic radiation improves local control after hysterectomy for uterine leiomyosarcoma: a 20-year experience. Int J Gynecol Cancer. 2009;19:1080–4. - PubMed
-
- Cuevas C, Francesch A. Development of Yondelis (trabectedin, ET-743). A semisynthetic process solves the supply problem. Nat Prod Rep. 2009;26:322–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

