Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease
- PMID: 21996293
- PMCID: PMC3308100
- DOI: 10.1186/ar3486
Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease
Abstract
Introduction: T cell abnormalities have been associated with the pathogenesis of systemic sclerosis (SSc). Recently, besides T helper (Th)17 cells, the Th22 subset has been identified in humans. Our purpose was to investigate the pattern of cytokines produced and chemokine-receptors expressed by peripheral blood (PB) Th cells in SSc and healthy donors (HD) focusing on cells producing interleukin (IL)-17 and IL-22 and to identify specific clinical associations.
Methods: Clinical data and peripheral blood were collected in 33 SSc individuals and 29 HD. IL-17A, IL-22, interferon gamma (IFN-γ), IL-4 production, the chemokine receptors CCR4, CCR6, CCR10, CXCR3 expression and the CD161 Th17 cell marker were assessed by multiparametric flow cytometry in PB CD4+ T cells. Intracellular cytokine accumulation was further investigated in CD4+ T cells expanded in vitro for seven days.
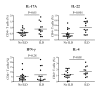
Results: The frequency of Th22, Th17, Th2, but not Th1 cells, was significantly increased in SSc individuals compared to HD. The percentage of CD161+CD4+ T cells was increased in SSc and correlated with the percentage of IL-17A producing cells. Moreover, the expression of the skin- and lung-homing chemokine receptor CCR6 correlated with the frequency of IL-22 and IL-17A-producing cells in SSc but not in HD. Finally, SSc interstitial lung disease (ILD) was strongly associated with higher numbers of IL-22 and, to a lesser extent, IL-17A-producing cells.
Conclusions: IL-22 and IL-17A-producing T cells with skin- and lung-homing capabilities are characteristically increased in SSc. These findings support the hypothesis that Th22, in addition to Th17 cells, may be involved in pathological processes leading to SSc. While the association between IL-22 producing cells and ILD needs to be assessed in larger cohorts of patients, the increased frequency of Th22 cells appears to be a useful novel biomarker in SSc.
Figures

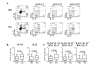
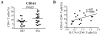
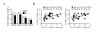
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

