Polyinosinic:polycytidylic acid induces protein kinase D-dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells
- PMID: 21996340
- PMCID: PMC3273326
- DOI: 10.1016/j.jaci.2011.08.035
Polyinosinic:polycytidylic acid induces protein kinase D-dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells
Abstract
Background: Disruption of the epithelial barrier might be a risk factor for allergen sensitization and asthma. Viral respiratory tract infections are strongly associated with asthma exacerbation, but the effects of respiratory viruses on airway epithelial barrier function are not well understood. Many viruses generate double-stranded RNA, which can lead to airway inflammation and initiate an antiviral immune response.
Objectives: We investigated the effects of the synthetic double-stranded RNA polyinosinic:polycytidylic acid (polyI:C) on the structure and function of the airway epithelial barrier in vitro.
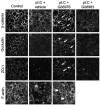
Methods: 16HBE14o- human bronchial epithelial cells and primary airway epithelial cells at an air-liquid interface were grown to confluence on Transwell inserts and exposed to polyI:C. We studied epithelial barrier function by measuring transepithelial electrical resistance and paracellular flux of fluorescent markers and structure of epithelial apical junctions by means of immunofluorescence microscopy.
Results: PolyI:C induced a profound decrease in transepithelial electrical resistance and increase in paracellular permeability. Immunofluorescence microscopy revealed markedly reduced junctional localization of zonula occludens-1, occludin, E-cadherin, β-catenin, and disorganization of junction-associated actin filaments. PolyI:C induced protein kinase D (PKD) phosphorylation, and a PKD antagonist attenuated polyI:C-induced disassembly of apical junctions and barrier dysfunction.
Conclusions: PolyI:C has a powerful and previously unsuspected disruptive effect on the airway epithelial barrier. PolyI:C-dependent barrier disruption is mediated by disassembly of epithelial apical junctions, which is dependent on PKD signaling. These findings suggest a new mechanism potentially underlying the associations between viral respiratory tract infections, airway inflammation, and allergen sensitization.
Copyright © 2011 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: L. A. Beck receives research support from Centocor, Regeneron, and Genentech. S. N. Georas receives research support from the National Institutes of Health. The rest of the authors declare that they have no relevant conflicts of interest.
Figures






Comment in
-
Plasticity of airway epithelial cells.J Allergy Clin Immunol. 2011 Dec;128(6):1225-6. doi: 10.1016/j.jaci.2011.10.006. J Allergy Clin Immunol. 2011. PMID: 22133319 Free PMC article. No abstract available.
References
-
- Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

