Leukocyte compartments in the mouse lung: distinguishing between marginated, interstitial, and alveolar cells in response to injury
- PMID: 21996427
- PMCID: PMC3328189
- DOI: 10.1016/j.jim.2011.09.013
Leukocyte compartments in the mouse lung: distinguishing between marginated, interstitial, and alveolar cells in response to injury
Abstract
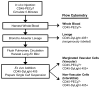
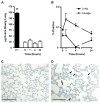
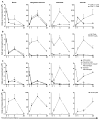
We developed a flow cytometry-based assay to simultaneously quantify multiple leukocyte populations in the marginated vascular, interstitial, and alveolar compartments of the mouse lung. An intravenous injection of a fluorescently labeled anti-CD45 antibody was used to label circulating and marginated vascular leukocytes. Following vascular flushing to remove non-adherent cells and collection of broncho-alveolar lavage (BAL) fluid, lungs were digested and a second fluorescent anti-CD45 antibody was added ex vivo to identify cells not located in the vascular space. In the naïve mouse lung, we found about 11 million CD45+ leukocytes, of which 87% (9.5 million) were in the vascular marginated compartment, consisting of 17% NK cells, 17% neutrophils, 57% mononuclear myeloid cells (monocytes, macrophage precursors and dendritic cells), and 10% T cells (CD4+, CD8+, and invariant NKT cells). Non-vascular compartments including the interstitial compartment contained 7.7×10(5)cells, consisting of 49% NK cells, 25% dendritic cells, and 16% other mononuclear myeloid cells. The alveolar compartment was overwhelmingly populated by macrophages (5.63×10(5)cells, or 93%). We next studied leukocyte margination and extravasation into the lung following acid injury, a model of gastric aspiration. At 1 h after injury, neutrophils were markedly elevated in the blood while all other circulating leukocytes declined by an average of 79%. At 4 h after injury, there was a peak in the numbers of marginated neutrophils, NK cells, CD4+ and CD8+ T cells and a peak in the number of alveolar NK cells. Most interstitial cells consisted of DCs, neutrophils, and CD4+ T cells, and most alveolar compartment cells consisted of macrophages, neutrophils, and NK cells. At 24 h after injury, there was a decline in the number of all marginated and interstitial leukocytes and a peak in alveolar neutrophils. In sum, we have developed a novel assay to study leukocyte margination and trafficking following pulmonary inflammation and show that marginated cells comprise a large fraction of lung leukocytes that increases shortly after lung injury. This assay may be of interest in future studies to determine if leukocytes become activated upon adherence to the endothelium, and have properties that distinguish them from interstitial and circulating cells.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

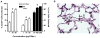
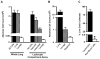
References
6.1. Journal References
-
- Basse PH, Hokland P, Gundersen HJ, Hokland M. Enumeration of organ-associated natural killer cells in mice: application of a new stereological method. APMIS. 1992;100:202–8. - PubMed
6.2 Web References
-
- The Jackson Laboratory. Mouse Facts, Mouse Genome Informatics (MGI) Web Site. 2011 Mar 29; World Wide Web (URL: http://www.informatics.jax.org/mgihome/other/mouse_facts1.shtml)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

