Optimizing dynamic interactions between a cardiac patch and inflammatory host cells
- PMID: 21996612
- PMCID: PMC3325606
- DOI: 10.1159/000331392
Optimizing dynamic interactions between a cardiac patch and inflammatory host cells
Abstract
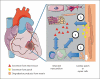
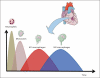
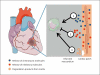
Damaged heart muscle has only a minimal ability for regeneration following myocardial infarction in which cardiomyocytes are lost to ischemia. The most clinically promising approach to regeneration of cardiac muscle currently under investigation is that of injecting cardiogenic repair cells or implanting a preformed tissue-engineered patch. While major advances are being made in the derivation of functional human cardiomyocytes and the development of tissue-engineering modalities for cardiac repair, the host environment into which the repair cells are placed is largely overlooked. Within seconds of myocardial ischemia, hypoxia sets in in the myocardium and the inflammatory response starts, characterized by rapid deployment of circulating cells and the release of paracrine and autocrine signals. Therefore, the inflammatory conditions under which these interactions take place, the design of the scaffold material used, and the maturity of the implanted cells will determine the outcomes of any stem cell-based therapy. We discuss here the interactions between implanted and inflammatory cells of the host, which are critical for the design of effective heart repair therapies.
Copyright © 2011 S. Karger AG, Basel.
Figures


References
-
- Abarbanell A.M., Herrmann J.L., Weil B.R., Wang Y., Tan J., Moberly S.P., Fiege J.W., Meldrum D.R. Animal models of myocardial and vascular injury. J Surg Res. 2010;162:239–249. - PubMed
-
- Ascher N.L., Hoffman R.A., Hanto D.W., Simmons R.L. Cellular basis of allograft rejection. Immunol Rev. 1984;77:217–232. - PubMed
-
- Atluri P., Woo Y.J. Pro-angiogenic cytokines as cardiovascular therapeutics: assessing the potential. BioDrugs. 2008;22:209–222. - PubMed
-
- Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. - PubMed
-
- Badylak S.F., Freytes D.O., Gilbert T.W. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1–13. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

