Snail1 mediates hypoxia-induced melanoma progression
- PMID: 21996677
- PMCID: PMC3260799
- DOI: 10.1016/j.ajpath.2011.08.038
Snail1 mediates hypoxia-induced melanoma progression
Abstract
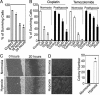
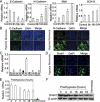
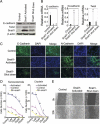
Tumor hypoxia is a known adverse prognostic factor, and the hypoxic dermal microenvironment participates in melanomagenesis. High levels of hypoxia inducible factor (HIF) expression in melanoma cells, particularly HIF-2α, is associated with poor prognosis. The mechanism underlying the effect of hypoxia on melanoma progression, however, is not fully understood. We report evidence that the effects of hypoxia on melanoma cells are mediated through activation of Snail1. Hypoxia increased melanoma cell migration and drug resistance, and these changes were accompanied by increased Snail1 and decreased E-cadherin expression. Snail1 expression was regulated by HIF-2α in melanoma. Snail1 overexpression led to more aggressive tumor phenotypes and features associated with stem-like melanoma cells in vitro and increased metastatic capacity in vivo. In addition, we found that knockdown of endogenous Snail1 reduced melanoma proliferation and migratory capacity. Snail1 knockdown also prevented melanoma metastasis in vivo. In summary, hypoxia up-regulates Snail1 expression and leads to increased metastatic capacity and drug resistance in melanoma cells. Our findings support that the effects of hypoxia on melanoma are mediated through Snail1 gene activation and suggest that Snail1 is a potential therapeutic target for the treatment of melanoma.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
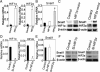


References
-
- Greenlee R.T., Murray T., Bolden S., Wingo P.A. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. - PubMed
-
- Wingo P.A., Ries L.A., Giovino G.A., Miller D.S., Rosenberg H.M., Shopland D.R., Thun M.J., Edwards B.K. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91:675–690. - PubMed
-
- Rigel D.S., Carucci J.A. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA Cancer J Clin. 2000;50:215–236. - PubMed
-
quiz 237–240
-
- Brizel D.M., Scully S.P., Harrelson J.M., Layfield L.J., Bean J.M., Prosnitz L.R., Dewhirst M.W. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

