Plasticity of astroglial networks in olfactory glomeruli
- PMID: 21997206
- PMCID: PMC3214998
- DOI: 10.1073/pnas.1107386108
Plasticity of astroglial networks in olfactory glomeruli
Abstract
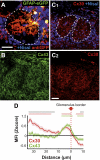
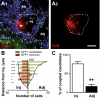
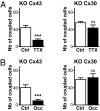
Several recent findings have shown that neurons as well as astrocytes are organized into networks. Indeed, astrocytes are interconnected through connexin-formed gap junction channels allowing exchanges of ions and signaling molecules. The aim of this study is to characterize astrocyte network properties in mouse olfactory glomeruli where neuronal connectivity is highly ordered. Dye-coupling experiments performed in olfactory bulb acute slices (P16-P22) highlight a preferential communication between astrocytes within glomeruli and not between astrocytes in adjacent glomeruli. Such organization relies on the oriented morphology of glomerular astrocytes to the glomerulus center and the enriched expression of two astroglial connexins (Cx43 and Cx30) within the glomeruli. Glomerular astrocytes detect neuronal activity showing membrane potential fluctuations correlated with glomerular local field potentials. Accordingly, gap junctional coupling of glomerular networks is reduced when neuronal activity is silenced by TTX treatment or after early sensory deprivation. Such modulation is lost in Cx30 but not in Cx43 KO mice, indicating that Cx30-formed channels are the molecular targets of this activity-dependent modulation. Extracellular potassium is a key player in this neuroglial interaction, because (i) the inhibition of dye coupling observed in the presence of TTX or after sensory deprivation is restored by increasing [K(+)](e) and (ii) treatment with a K(ir) channel blocker inhibits dye spread between glomerular astrocytes. Together, these results demonstrate that extracellular potassium generated by neuronal activity modulates Cx30-mediated gap junctional communication between glomerular astrocytes, indicating that strong neuroglial interactions take place at this first relay of olfactory information processing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mori K, Nagao H, Yoshihara Y. The olfactory bulb: Coding and processing of odor molecule information. Science. 1999;286:711–715. - PubMed
-
- Mombaerts P, et al. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. - PubMed
-
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. - PubMed
-
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

