Hops (Humulus lupulus) inhibits oxidative estrogen metabolism and estrogen-induced malignant transformation in human mammary epithelial cells (MCF-10A)
- PMID: 21997247
- PMCID: PMC3252489
- DOI: 10.1158/1940-6207.CAPR-11-0348
Hops (Humulus lupulus) inhibits oxidative estrogen metabolism and estrogen-induced malignant transformation in human mammary epithelial cells (MCF-10A)
Abstract
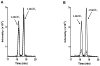
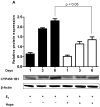
Long-term exposure to estrogens including those in traditional hormone replacement therapy (HRT) increases the risk of developing hormone-dependent cancers. As a result, women are turning to over-the-counter (OTC) botanical dietary supplements, such as black cohosh (Cimicifuga racemosa) and hops (Humulus lupulus), as natural alternatives to HRT. The two major mechanisms which likely contribute to estrogen and/or HRT cancer risk are: the estrogen receptor-mediated hormonal pathway; and the chemical carcinogenesis pathway involving formation of estrogen quinones that damage DNA and proteins, hence initiating and promoting carcinogenesis. Because, OTC botanical HRT alternatives are in widespread use, they may have the potential for chemopreventive effects on estrogen carcinogenic pathways in vivo. Therefore, the effect of OTC botanicals on estrogen-induced malignant transformation of MCF-10A cells was studied. Cytochrome P450 catalyzed hydroxylation of estradiol at the 4-position leads to an o-quinone believed to act as the proximal carcinogen. Liquid chromatography/tandem mass spectrometry analysis of estradiol metabolites showed that 4-hydroxylation was inhibited by hops, whereas black cohosh was without effect. Estrogen-induced expression of CYP450 1B1 and CYP450 1A1 was attenuated by the hops extract. Two phenolic constituents of hops (xanthohumol, XH; 8-prenylnaringenin, 8-PN) were tested: 8-PN was a potent inhibitor, whereas XH had no effect. Finally, estrogen-induced malignant transformation of MCF-10A cells was observed to be significantly inhibited by hops (5 μg/mL) and 8-PN (50 nmol/L). These data suggest that hops extracts possess cancer chemopreventive activity through attenuation of estrogen metabolism mediated by 8-PN.
©2011 AACR.
Figures






References
-
- Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J Steroid Biochem Mol Biol. 2003;87:1–25. - PubMed
-
- Bolton JL. Mechanisms of Estrogen Carcinogenesis: Modulation by Botanical Natural Products. In: Penning TM, editor. Chemical Carcinogenesis. Humana Press; 2011. pp. 75–93.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

