Mutability of prions
- PMID: 21997293
- PMCID: PMC3245691
- DOI: 10.1038/embor.2011.191
Mutability of prions
Abstract
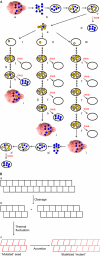
Murine prions transferred from brain to cultured cells gradually adapt to the new environment. Brain-derived 22L prions can infect neuroblastoma-derived PK1 cells in the presence of swainsonine (swa); that is, they are 'swa resistant'. PK1 cell-adapted 22L prions are swa sensitive; however, propagation in swa results in selection of swa-resistant substrains. Cloned, PK1 cell-adapted 22L prions were initially unable to develop swa resistance ('swa incompetent'); however, after serial propagation for 30-90 doublings, four of nine clones became swa competent, showing that swa-resistant 'mutants' arose during replication. Mutations in the case of prions are attributed to heritable changes in PrP(Sc) conformation. One clone remained swa incompetent even after 10(35)-fold expansion; surprisingly, after propagation in brain, it yielded swa-resistant prions, indistinguishable from the original 22L population. Thus, cell-adapted 22L prions assumed either mutable or virtually immutable conformations; however, when passaged through the brain all became mutable. Mutability is thus a substrain-specific attribute.
Conflict of interest statement
The authors have no conflict of interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

