Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis
- PMID: 21997369
- PMCID: PMC3359483
- DOI: 10.4161/auto.7.11.17660
Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis
Abstract
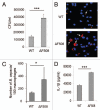
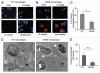
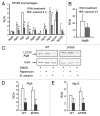
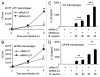
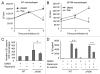
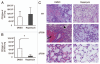
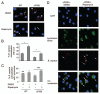
Cystic fibrosis (CF) is the most common inherited lethal disease of Caucasians which results in multi organ dysfunction. However, 85% of the deaths are due to pulmonary infections. Infection by Burkholderia cenocepacia (B. cepacia) is a particularly lethal threat to CF patients because it causes severe and persistent lung inflammation and is resistant to nearly all available antibiotics. In CFTR ΔF508 mouse macrophages, B. cepacia persists in vacuoles that do not fuse with the lysosomes and mediates increased production of IL-1β. It is believed that intracellular bacterial survival contributes to the persistence of the bacterium. Here we show for the first time that in wild-type macrophages but not in ΔF508 macrophages, many B. cepacia reside in autophagosomes that fuse with lysosomes at later stages of infection. Accordingly, association and intracellular survival of B. cepacia are higher in CFTR-ΔF508 (ΔF508) macrophages than in WT macrophages. An autophagosome is a compartment that engulfs non-functional organelles and parts of the cytoplasm then delivers them to the lysosome for degradation to produce nutrients during periods of starvation or stress. Furthermore, we show that B. cepacia downregulates autophagy genes in WT and ΔF508 macrophages. However, autophagy dysfunction is more pronounced in ΔF508 macrophages since they already have compromised autophagy activity. We demonstrate that the autophagy-stimulating agent, rapamycin markedly decreases B. cepacia infection in vitro by enhancing the clearance of B. cepacia via induced autophagy. In vivo, Rapamycin decreases bacterial burden in the lungs of CF mice and drastically reduces signs of lung inflammation. Together, our studies reveal that if efficiently activated, autophagy can control B. cepacia infection and ameliorate the associated inflammation. Therefore, autophagy is a novel target for new drug development for CF patients to control B. cepacia infection and accompanying inflammation.
Figures
Comment in
-
Autophagy and the evasion of host defense: a new variation on the theme for Burkholderia cepacia?Autophagy. 2011 Nov;7(11):1269-70. doi: 10.4161/auto.7.11.17941. Epub 2011 Nov 1. Autophagy. 2011. PMID: 21997365 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
