Regulation of alveolar epithelial Na+ channels by ERK1/2 in chlorine-breathing mice
- PMID: 21997487
- PMCID: PMC3326429
- DOI: 10.1165/rcmb.2011-0309OC
Regulation of alveolar epithelial Na+ channels by ERK1/2 in chlorine-breathing mice
Abstract
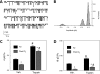
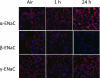
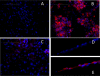
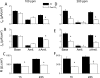
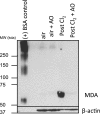
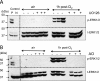
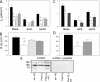
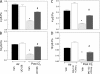
The mechanisms by which the exposure of mice to Cl(2) decreases vectorial Na(+) transport and fluid clearance across their distal lung spaces have not been elucidated. We examined the biophysical, biochemical, and physiological changes of rodent lung epithelial Na(+) channels (ENaCs) after exposure to Cl(2), and identified the mechanisms involved. We measured amiloride-sensitive short-circuit currents (I(amil)) across isolated alveolar Type II (ATII) cell monolayers and ENaC single-channel properties by patching ATII and ATI cells in situ. α-ENaC, γ-ENaC, total and phosphorylated extracellular signal-related kinase (ERK)1/2, and advanced products of lipid peroxidation in ATII cells were measured by Western blot analysis. Concentrations of reactive intermediates were assessed by electron spin resonance (ESR). Amiloride-sensitive Na(+) channels with conductances of 4.5 and 18 pS were evident in ATI and ATII cells in situ of air-breathing mice. At 1 hour and 24 hours after exposure to Cl(2), the open probabilities of these two channels decreased. This effect was prevented by incubating lung slices with inhibitors of ERK1/2 or of proteasomes and lysosomes. The exposure of ATII cell monolayers to Cl(2) increased concentrations of reactive intermediates, leading to ERK1/2 phosphorylation and decreased I(amil) and α-ENaC concentrations at 1 hour and 24 hours after exposure. The administration of antioxidants to ATII cells before and after exposure to Cl(2) decreased concentrations of reactive intermediates and ERK1/2 activation, which mitigated the decrease in I(amil) and ENaC concentrations. The reactive intermediates formed during and after exposure to Cl(2) activated ERK1/2 in ATII cells in vitro and in vivo, leading to decreased ENaC concentrations and activity.
Figures




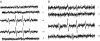
References
-
- Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 2002;82:569–600 - PubMed
-
- Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol 2009;71:403–423 - PubMed
-
- Burch LH, Talbot CR, Knowles MR, Canessa CM, Rossier BC, Boucher RC. Relative expression of the human epithelial Na+ channel subunits in normal and cystic fibrosis airways. Am J Physiol 1995;269:C511–C518 - PubMed
-
- Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 1999;61:627–661 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5U01ES015676-05/ES/NIEHS NIH HHS/United States
- HL097218/HL/NHLBI NIH HHS/United States
- R01 CA131653/CA/NCI NIH HHS/United States
- U54 ES017218/ES/NIEHS NIH HHS/United States
- 5U54ES017218-03/ES/NIEHS NIH HHS/United States
- HL105473/HL/NHLBI NIH HHS/United States
- R01 HL031197/HL/NHLBI NIH HHS/United States
- 5R01CA131653/CA/NCI NIH HHS/United States
- 5R01HL031197-25/HL/NHLBI NIH HHS/United States
- R21 HL097218/HL/NHLBI NIH HHS/United States
- R01 HL105473/HL/NHLBI NIH HHS/United States
- U01 ES015676/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

