Blood-brain barrier invasion by Cryptococcus neoformans is enhanced by functional interactions with plasmin
- PMID: 21998162
- PMCID: PMC3352358
- DOI: 10.1099/mic.0.051524-0
Blood-brain barrier invasion by Cryptococcus neoformans is enhanced by functional interactions with plasmin
Abstract
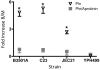
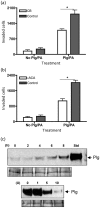
Cryptococcus neoformans can invade the central nervous system through diverse mechanisms. We examined a possible role for host plasma proteases in the neurotropic behaviour of this blood-borne fungal pathogen. Plasminogen is a plasma-enriched zymogen that can passively coat the surface of blood-borne pathogens and, upon conversion to the serine protease plasmin, facilitate pathogen dissemination by degrading vascular barriers. In this study, plasminogen-to-plasmin conversion on killed and viable hypoencapsulated strains of C. neoformans required the addition of plasminogen activator (PA), but this conversion occurred in the absence of supplemented PA when viable strains were cultured with brain microvascular endothelial cells (BMEC). Plasmin-coated C. neoformans showed an enhanced invasive ability in Matrigel invasion assays that was significantly augmented in the presence of BMEC. The invasive effect of plasmin required viable pathogen and correlated with rapid declines in BMEC barrier function. Plasmin-enhanced invasion was inhibited by aprotinin, carboxypeptidase B, the lysine analogue epsilon-aminocaproic acid, and by capsule development. C. neoformans caused plasminogen-independent declines in BMEC barrier function that were associated with pathogen-induced host damage; however, such declines were significantly delayed and less extensive than those observed with plasmin-coated pathogen. BMEC adhesion and damage by hypoencapsulated C. neoformans were diminished by capsule induction but unaltered by plasminogen and/or PA. We conclude that hypoencapsulated C. neoformans can invade BMEC by a plasmin-dependent mechanism, in vitro, and that small, or minimal, surface capsule expression during the blood-borne phase of cryptococcosis may promote virulence by means of plasmin(ogen) acquisition.
Figures

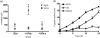


References
-
- Ambesi A., Klein R. M., Pumiglia K. M., McKeown-Longo P. J. (2005). Anastellin, a fragment of the first type III repeat of fibronectin, inhibits extracellular signal-regulated kinase and causes G1 arrest in human microvessel endothelial cells. Cancer Res 65, 148–156. - PubMed
-
- Bennett J. E., Kwon-Chung K. J., Howard D. H. (1977). Epidemiologic differences among serotypes of Cryptococcus neoformans. Am J Epidemiol 105, 582–586. - PubMed
-
- Bergmann S., Hammerschmidt S. (2007). Fibrinolysis and host response in bacterial infections. Thromb Haemost 98, 512–520. - PubMed
-
- Bolaños B., Mitchell T. G. (1989). Phagocytosis and killing of Cryptococcus neoformans by rat alveolar macrophages in the absence of serum. J Leukoc Biol 46, 521–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

