Function of the acrosomal matrix: zona pellucida 3 receptor (ZP3R/sp56) is not essential for mouse fertilization
- PMID: 21998167
- PMCID: PMC3313668
- DOI: 10.1095/biolreprod.111.095877
Function of the acrosomal matrix: zona pellucida 3 receptor (ZP3R/sp56) is not essential for mouse fertilization
Abstract
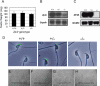
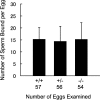
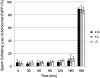
In mammalian fertilization, sperm-zona pellucida binding is considered to be a critical aspect of gamete interaction. In this study, we examine the mouse sperm acrosomal matrix protein zona pellucida 3 receptor (ZP3R; formerly called sp56) because of our interest in defining the function of the acrosomal matrix, the particulate compartment within the sperm secretory acrosome. Using targeted deletion of the Zp3r gene by homologous recombination, we examined the fertility of nullizygous animals. Our experiments showed that males and females homozygous for the affected gene exhibited no differences in litter sizes compared to wild-type and heterozygous animals. Testis weights of nullizygous males were equivalent to those of wild-type and heterozygous males, and no differences in the number of sperm produced by mice of three genotypes were found. In vitro fertilization rates using cumulus-intact and cumulus-free oocytes were also equivalent. Examination of sperm-binding zonae of unfertilized eggs and the ability of the sperm to undergo acrosomal exocytosis in response to calcium ionophore A23187 displayed no differences between wild-type, heterozygous, and nullizygous mouse sperm. These results provide further evidence that either ZP3R is not involved in sperm-zona pellucida binding or this process might be functionally redundant, involving multiple proteins for gamete interactions.
Figures


References
-
- Tulsiani DR, Yoshida-Komiya H, Araki Y. Mammalian fertilization: a carbohydrate-mediated event. Biol Reprod 1997; 57: 487 494 - PubMed
-
- Clark GF. Molecular models for mouse sperm-oocyte binding. Glycobiology 2011; 21: 3 5 - PubMed
-
- Baba T, Azuma S, Kashiwabara S, Toyoda Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem 1994; 269: 31845 31849 - PubMed
-
- Lu Q, Shur BD. Sperm from beta-1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development 1997; 124: 4121 4131 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

