Hook2 is involved in the morphogenesis of the primary cilium
- PMID: 21998199
- PMCID: PMC3226474
- DOI: 10.1091/mbc.E11-05-0405
Hook2 is involved in the morphogenesis of the primary cilium
Abstract
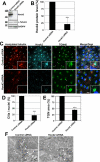
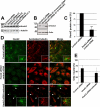
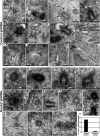
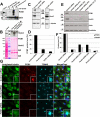
Primary cilia originate from the centrosome and play essential roles in several cellular, developmental, and pathological processes, but the underlying mechanisms of ciliogenesis are not fully understood. Given the involvement of the adaptor protein Hook2 in centrosomal homeostasis and protein transport to pericentrosomal aggresomes, we explored its role in ciliogenesis. We found that in human retinal epithelial cells, Hook2 localizes at the Golgi apparatus and centrosome/basal body, a strategic partitioning for ciliogenesis. Of importance, Hook2 depletion disrupts ciliogenesis at a stage before the formation of the ciliary vesicle at the distal tip of the mother centriole. Using two hybrid and immunoprecipitation assays and a small interfering RNA strategy, we found that Hook2 interacts with and stabilizes pericentriolar material protein 1 (PCM1), which was reported to be essential for the recruitment of Rab8a, a GTPase that is believed to be crucial for membrane transport to the primary cilium. Of interest, GFP::Rab8a coimmunoprecipitates with endogenous Hook2 and PCM1. Finally, GFP::Rab8a can overcome Hook2 depletion, demonstrating a functional interaction between Hook2 and these two important regulators of ciliogenesis. The data indicate that Hook2 interacts with PCM1 in a complex that also contains Rab8a and regulates a limiting step required for further initiation of ciliogenesis after centriole maturation.
Figures


References
-
- Alieva IB, Vorobjev IA. Vertebrate primary cilia: a sensory part of centrosomal complex in tissue cells, but a “sleeping beauty” in cultured cells? Cell Biol Int. 2004;28:139–150. - PubMed
-
- den Hollander AI, et al. Mutations in LCA5, encoding the ciliary protein lebercilin, cause Leber congenital amaurosis. Nat Genet. 2007;39:889–895. - PubMed
-
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

