Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules
- PMID: 21998204
- PMCID: PMC3226476
- DOI: 10.1091/mbc.E11-07-0616
Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules
Abstract
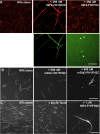
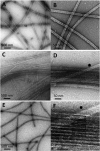
A number of cellular processes use both microtubules and actin filaments, but the molecular machinery linking these two cytoskeletal elements remains to be elucidated in detail. Formins are actin-binding proteins that have multiple effects on actin dynamics, and one formin, mDia2, has been shown to bind and stabilize microtubules through its formin homology 2 (FH2) domain. Here we show that three formins, INF2, mDia1, and mDia2, display important differences in their interactions with microtubules and actin. Constructs containing FH1, FH2, and C-terminal domains of all three formins bind microtubules with high affinity (K(d) < 100 nM). However, only mDia2 binds microtubules at 1:1 stoichiometry, with INF2 and mDia1 showing saturating binding at approximately 1:3 (formin dimer:tubulin dimer). INF2-FH1FH2C is a potent microtubule-bundling protein, an effect that results in a large reduction in catastrophe rate. In contrast, neither mDia1 nor mDia2 is a potent microtubule bundler. The C-termini of mDia2 and INF2 have different functions in microtubule interaction, with mDia2's C-terminus required for high-affinity binding and INF2's C-terminus required for bundling. mDia2's C-terminus directly binds microtubules with submicromolar affinity. These formins also differ in their abilities to bind actin and microtubules simultaneously. Microtubules strongly inhibit actin polymerization by mDia2, whereas they moderately inhibit mDia1 and have no effect on INF2. Conversely, actin monomers inhibit microtubule binding/bundling by INF2 but do not affect mDia1 or mDia2. These differences in interactions with microtubules and actin suggest differential function in cellular processes requiring both cytoskeletal elements.
Figures






References
-
- Al-Bassam J, Roger B, Halpain S, Milligan RA. Analysis of the weak interactions of ADP-Unc104 and ADP-kinesin with microtubules and their inhibition by MAP2c. Cell Motil Cytoskeleton. 2007;64:377–389. - PubMed
-
- Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac MH. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol. 2008;18:1514–1519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

